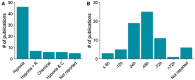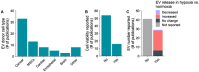Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions - PubMed (original) (raw)
Review
Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions
Nea Bister et al. J Extracell Vesicles. 2020 Oct.
Abstract
Hypoxia is an essential hallmark of several serious diseases such as cardiovascular and metabolic disorders and cancer. A decline in the tissue oxygen level induces hypoxic responses in cells which strive to adapt to the changed conditions. A failure to adapt to prolonged or severe hypoxia can trigger cell death. While some cell types, such as neurons, are highly vulnerable to hypoxia, cancer cells take advantage of a hypoxic environment to undergo tumour growth, angiogenesis and metastasis. Hypoxia-induced processes trigger complex intercellular communication and there are now indications that extracellular vesicles (EVs) play a fundamental role in these processes. Recent developments in EV isolation and characterization methodology have increased the awareness of the importance of EV purity in functional and cargo studies. Cell death, a hallmark of severe hypoxia, is a known source of intracellular contaminants in isolated EVs. In this review, methodological aspects of studies investigating hypoxia-induced EVs are critically evaluated. Key concerns and gaps in the current knowledge are highlighted and future directions for studies are set. To accelerate and advance research, an in-depth analysis of the functions and cargo of hypoxic EVs, compared to normoxic EVs, is provided with the focus on the altered microRNA contents of the EVs.
Keywords: cancer; exosomes; hypoxia; infarction; intercellular communication; mesenchymal stem cells; microRNA.
© 2020 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures
FIGURE 1
Extracellular vesicle (EV) biogenesis and the properties of hypoxia induced EVs in the recipient cells. Exosomes are formed inside multivesicular bodies (MVBs) by inward budding of the endosomal membrane, leading to the release of small vesicles inside the endosome. MVBs fuse with the plasma membrane to release exosomes to the extracellular space while microvesicles form directly from the outward budding of the plasma membrane. Both EV types contain a variety of cargos. EVs released by hypoxic cells have several functions when they reach the recipient cells. Figure was created with BioRender.com
FIGURE 2
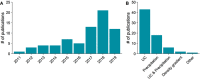
Number of publications on hypoxia induced extracellular vesicles (EVs) is rapidly accumulating and shows significant heterogeneity in the EV isolation methods used. Number of new publications on hypo‐EVs has been increasing during the last few years (a). Numbers are based on PubMed searches using “extracellular vesicle”, “exosome” or “microvesicle” and “hypoxia” or “ischemia” search words and screening for suitable articles based on the title which yielded 70 original publications. In these publications, different techniques were used for EV isolation, most commonly involving ultracentrifugation (UC) or precipitation‐based methodologies (b). Gradient refers to the use of an iodixanol or sucrose (or some other agent) density gradient step in the UC purification
FIGURE 3
Experimental set‐ups differ by the method of hypoxia induction and the length of this exposure. Most studies were carried out using a hypoxic incubator with low oxygen levels (a). Some studies used both hypoxia and chemical hypoxia. The duration of hypoxia was commonly between 24 and 48 h (b). For reoxygenation studies, the duration of reoxygenation is plotted. R = reoxygenation, C = chemical
FIGURE 4
Effect of hypoxia on extracellular vesicle (EV) release. The effect of hypoxia on the release of EVs has been studied in the context of several different EV producing cell types (a). Only 1/5 of publications reported the effect of hypoxia induction on the viability of the EV producing cells (b). Studies reporting quantitative effects on EV release reported mainly increased EV release during hypoxia, or no change (c)
FIGURE 5
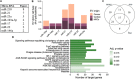
The release of several hypoxia‐related miRNAs is increased in hypoxic extracellular vesicles (EVs). Elevated levels of seven miRNAs have been reported to be released in EVs under hypoxia in more than one publication (a total of 19 publications) (a). In these included studies, EVs were isolated from several donor cell types and the number of publications reporting an increase of specific miRNAs in hypo‐EVs is depicted (b). Separate pathway analysis for each species in the studies, using target genes of altered miRNAs, showed 17 overlapping pathways between human (hsa), mouse (mmu) and rat (rno) (c). These overlapping, significantly enriched, pathways were related to important processes for cell survival in hypoxia (such as FoxO, HIF‐1, MAPK and PI3K‐Akt signalling pathways) and cancer progression (d)
Similar articles
- Hypoxic bone marrow mesenchymal cell-extracellular vesicles containing miR-328-3p promote lung cancer progression via the NF2-mediated Hippo axis.
Liu X, Jiang F, Wang Z, Tang L, Zou B, Xu P, Yu T. Liu X, et al. J Cell Mol Med. 2021 Jan;25(1):96-109. doi: 10.1111/jcmm.15865. Epub 2020 Nov 21. J Cell Mol Med. 2021. PMID: 33219752 Free PMC article. - Effect of hypoxia on extracellular vesicles in malignant and non-malignant conditions.
Niazi V, Ghafouri-Fard S. Niazi V, et al. Cancer Treat Res Commun. 2025;43:100924. doi: 10.1016/j.ctarc.2025.100924. Epub 2025 Apr 7. Cancer Treat Res Commun. 2025. PMID: 40209539 Review. - Hypoxic regulation of extracellular vesicles: Implications for cancer therapy.
Yoo S, Choi S, Kim I, Kim IS. Yoo S, et al. J Control Release. 2023 Nov;363:201-220. doi: 10.1016/j.jconrel.2023.09.034. Epub 2023 Sep 28. J Control Release. 2023. PMID: 37739015 Review. - Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer.
Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR, Di Vizio D. Ciardiello C, et al. Int J Mol Sci. 2016 Feb 6;17(2):175. doi: 10.3390/ijms17020175. Int J Mol Sci. 2016. PMID: 26861306 Free PMC article. Review. - Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia.
Sanz-Rubio D, Khalyfa A, Qiao Z, Ullate J, Marin JM, Kheirandish-Gozal L, Gozal D. Sanz-Rubio D, et al. Int J Mol Sci. 2021 May 25;22(11):5604. doi: 10.3390/ijms22115604. Int J Mol Sci. 2021. PMID: 34070558 Free PMC article.
Cited by
- Enhancing the Cellular Production of Extracellular Vesicles for Developing Therapeutic Applications.
Erwin N, Serafim MF, He M. Erwin N, et al. Pharm Res. 2023 Apr;40(4):833-853. doi: 10.1007/s11095-022-03420-w. Epub 2022 Nov 1. Pharm Res. 2023. PMID: 36319886 Free PMC article. Review. - Exosomes: a significant medium for regulating drug resistance through cargo delivery.
Ren B, Li X, Zhang Z, Tai S, Yu S. Ren B, et al. Front Mol Biosci. 2024 Jul 29;11:1379822. doi: 10.3389/fmolb.2024.1379822. eCollection 2024. Front Mol Biosci. 2024. PMID: 39135913 Free PMC article. Review. - 3D-hUMSCs Exosomes Ameliorate Vitiligo by Simultaneously Potentiating Treg Cells-Mediated Immunosuppression and Suppressing Oxidative Stress-Induced Melanocyte Damage.
Wang Q, Guo W, Niu L, Zhou Y, Wang Z, Chen J, Chen J, Ma J, Zhang J, Jiang Z, Wang B, Zhang Z, Li C, Jian Z. Wang Q, et al. Adv Sci (Weinh). 2024 Aug;11(31):e2404064. doi: 10.1002/advs.202404064. Epub 2024 Jun 17. Adv Sci (Weinh). 2024. PMID: 38887870 Free PMC article. - Understanding molecular characteristics of extracellular vesicles derived from different types of mesenchymal stem cells for therapeutic translation.
Ding Z, Greenberg ZF, Serafim MF, Ali S, Jamieson JC, Traktuev DO, March K, He M. Ding Z, et al. Extracell Vesicle. 2024 Jun;3:100034. doi: 10.1016/j.vesic.2024.100034. Epub 2024 Mar 2. Extracell Vesicle. 2024. PMID: 38957857 Free PMC article. - Revisiting the Mesenchymal "Stem vs. Stromal" Cell Dichotomy and Its Implications for Development of Improved Potency Metrics.
Phinney DG, Hwa Lee R, Boregowda SV. Phinney DG, et al. Stem Cells. 2023 May 15;41(5):444-452. doi: 10.1093/stmcls/sxad019. Stem Cells. 2023. PMID: 36891977 Free PMC article. Review.
References
- Bang, C. , Batkai, S. , Dangwal, S. , Gupta, S. K. , Foinquinos, A. , Holzmann, A. , … Thum, T. (2014). Cardiac fibroblast‐derived microRNA passenger strand‐enriched exosomes mediate cardiomyocyte hypertrophy. Journal of Clinical Investigation, 124(5), 2136–2146. 10.1172/JCI70577 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical