Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK - PubMed (original) (raw)
Randomized Controlled Trial
. 2021 Jan 9;397(10269):99-111.
doi: 10.1016/S0140-6736(20)32661-1. Epub 2020 Dec 8.
Sue Ann Costa Clemens 2, Shabir A Madhi 3, Lily Y Weckx 4, Pedro M Folegatti 5, Parvinder K Aley 1, Brian Angus 5, Vicky L Baillie 6, Shaun L Barnabas 7, Qasim E Bhorat 8, Sagida Bibi 1, Carmen Briner 9, Paola Cicconi 5, Andrea M Collins 10, Rachel Colin-Jones 1, Clare L Cutland 6, Thomas C Darton 11, Keertan Dheda 12, Christopher J A Duncan 13, Katherine R W Emary 1, Katie J Ewer 5, Lee Fairlie 14, Saul N Faust 15, Shuo Feng 1, Daniela M Ferreira 10, Adam Finn 16, Anna L Goodman 17, Catherine M Green 18, Christopher A Green 19, Paul T Heath 20, Catherine Hill 14, Helen Hill 10, Ian Hirsch 21, Susanne H C Hodgson 5, Alane Izu 22, Susan Jackson 5, Daniel Jenkin 5, Carina C D Joe 5, Simon Kerridge 1, Anthonet Koen 22, Gaurav Kwatra 14, Rajeka Lazarus 23, Alison M Lawrie 5, Alice Lelliott 1, Vincenzo Libri 24, Patrick J Lillie 25, Raburn Mallory 21, Ana V A Mendes 26, Eveline P Milan 27, Angela M Minassian 5, Alastair McGregor 28, Hazel Morrison 5, Yama F Mujadidi 1, Anusha Nana 9, Peter J O'Reilly 1, Sherman D Padayachee 29, Ana Pittella 30, Emma Plested 1, Katrina M Pollock 31, Maheshi N Ramasamy 1, Sarah Rhead 1, Alexandre V Schwarzbold 32, Nisha Singh 1, Andrew Smith 33, Rinn Song 34, Matthew D Snape 1, Eduardo Sprinz 35, Rebecca K Sutherland 36, Richard Tarrant 18, Emma C Thomson 37, M Estée Török 38, Mark Toshner 39, David P J Turner 40, Johan Vekemans 21, Tonya L Villafana 21, Marion E E Watson 5, Christopher J Williams 41, Alexander D Douglas 5, Adrian V S Hill 5, Teresa Lambe 5, Sarah C Gilbert 5, Andrew J Pollard 42; Oxford COVID Vaccine Trial Group
Collaborators, Affiliations
- PMID: 33306989
- PMCID: PMC7723445
- DOI: 10.1016/S0140-6736(20)32661-1
Randomized Controlled Trial
Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK
Merryn Voysey et al. Lancet. 2021.
Erratum in
- Department of Error.
[No authors listed] [No authors listed] Lancet. 2021 Jan 9;397(10269):98. doi: 10.1016/S0140-6736(20)32720-3. Lancet. 2021. PMID: 33422262 Free PMC article. No abstract available.
Abstract
Background: A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), if deployed with high coverage, could contribute to the control of the COVID-19 pandemic. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials.
Methods: This analysis includes data from four ongoing blinded, randomised, controlled trials done across the UK, Brazil, and South Africa. Participants aged 18 years and older were randomly assigned (1:1) to ChAdOx1 nCoV-19 vaccine or control (meningococcal group A, C, W, and Y conjugate vaccine or saline). Participants in the ChAdOx1 nCoV-19 group received two doses containing 5 × 1010 viral particles (standard dose; SD/SD cohort); a subset in the UK trial received a half dose as their first dose (low dose) and a standard dose as their second dose (LD/SD cohort). The primary efficacy analysis included symptomatic COVID-19 in seronegative participants with a nucleic acid amplification test-positive swab more than 14 days after a second dose of vaccine. Participants were analysed according to treatment received, with data cutoff on Nov 4, 2020. Vaccine efficacy was calculated as 1 - relative risk derived from a robust Poisson regression model adjusted for age. Studies are registered at ISRCTN89951424 and ClinicalTrials.gov, NCT04324606, NCT04400838, and NCT04444674.
Findings: Between April 23 and Nov 4, 2020, 23 848 participants were enrolled and 11 636 participants (7548 in the UK, 4088 in Brazil) were included in the interim primary efficacy analysis. In participants who received two standard doses, vaccine efficacy was 62·1% (95% CI 41·0-75·7; 27 [0·6%] of 4440 in the ChAdOx1 nCoV-19 group vs71 [1·6%] of 4455 in the control group) and in participants who received a low dose followed by a standard dose, efficacy was 90·0% (67·4-97·0; three [0·2%] of 1367 vs 30 [2·2%] of 1374; pinteraction=0·010). Overall vaccine efficacy across both groups was 70·4% (95·8% CI 54·8-80·6; 30 [0·5%] of 5807 vs 101 [1·7%] of 5829). From 21 days after the first dose, there were ten cases hospitalised for COVID-19, all in the control arm; two were classified as severe COVID-19, including one death. There were 74 341 person-months of safety follow-up (median 3·4 months, IQR 1·3-4·8): 175 severe adverse events occurred in 168 participants, 84 events in the ChAdOx1 nCoV-19 group and 91 in the control group. Three events were classified as possibly related to a vaccine: one in the ChAdOx1 nCoV-19 group, one in the control group, and one in a participant who remains masked to group allocation.
Interpretation: ChAdOx1 nCoV-19 has an acceptable safety profile and has been found to be efficacious against symptomatic COVID-19 in this interim analysis of ongoing clinical trials.
Funding: UK Research and Innovation, National Institutes for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, Bill & Melinda Gates Foundation, Lemann Foundation, Rede D'Or, Brava and Telles Foundation, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midland's NIHR Clinical Research Network, and AstraZeneca.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Figure
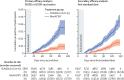
Kaplan-Meier cumulative incidence of primary symptomatic, NAAT-positive COVID-19 Cumulative incidence of symptomatic COVID-19 after two doses (left) or after first standard dose in participants receiving only standard-dose vaccines (right). Grey shaded areas show the exclusion period after each dose in which cases were excluded from the analysis. Blue and red shaded areas show 95% CIs. LD/SD=low-dose prime plus standard-dose boost. MenACWY=meningococcal group A, C, W, and Y conjugate vaccine. NAAT=nucleic acid amplification test. SD/SD=two standard-dose vaccines given.
Comment in
- Oxford COVID-vaccine paper highlights lingering unknowns about results.
Ledford H. Ledford H. Nature. 2020 Dec;588(7838):378-379. doi: 10.1038/d41586-020-03504-w. Nature. 2020. PMID: 33293710 No abstract available. - Oxford-AstraZeneca COVID-19 vaccine efficacy.
Knoll MD, Wonodi C. Knoll MD, et al. Lancet. 2021 Jan 9;397(10269):72-74. doi: 10.1016/S0140-6736(20)32623-4. Epub 2020 Dec 8. Lancet. 2021. PMID: 33306990 Free PMC article. No abstract available. - In adults, the Oxford/AstraZeneca vaccine had 70% efficacy against COVID-19 >14 d after the 2nd dose.
Chagla Z. Chagla Z. Ann Intern Med. 2021 Mar;174(3):JC29. doi: 10.7326/ACPJ202103160-029. Epub 2021 Mar 2. Ann Intern Med. 2021. PMID: 33646835 - Vaccines and SARS-CoV-2 variants: the urgent need for a correlate of protection.
Karim SSA. Karim SSA. Lancet. 2021 Apr 3;397(10281):1263-1264. doi: 10.1016/S0140-6736(21)00468-2. Epub 2021 Mar 23. Lancet. 2021. PMID: 33765410 Free PMC article. No abstract available. - Presenting severe adverse event data from clinical trials, and the need to prevent unblinding.
Armitage R. Armitage R. Public Health. 2021 Aug;197:e27. doi: 10.1016/j.puhe.2021.02.024. Epub 2021 Apr 2. Public Health. 2021. PMID: 33820618 No abstract available. - Safety and Considerations of the COVID-19 Vaccine Massive Deployment.
Li J, Song M, Guo D, Yi Y. Li J, et al. Virol Sin. 2021 Oct;36(5):1097-1103. doi: 10.1007/s12250-021-00408-5. Epub 2021 Jun 1. Virol Sin. 2021. PMID: 34061319 Free PMC article. No abstract available.
Similar articles
- Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Clutterbuck EA, Collins AM, Cutland CL, Darton TC, Dheda K, Dold C, Duncan CJA, Emary KRW, Ewer KJ, Flaxman A, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Galiza E, Goodman AL, Green CM, Green CA, Greenland M, Hill C, Hill HC, Hirsch I, Izu A, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Libri V, Lillie PJ, Marchevsky NG, Marshall RP, Mendes AVA, Milan EP, Minassian AM, McGregor A, Mujadidi YF, Nana A, Padayachee SD, Phillips DJ, Pittella A, Plested E, Pollock KM, Ramasamy MN, Ritchie AJ, Robinson H, Schwarzbold AV, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, White T, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Voysey M, et al. Lancet. 2021 Mar 6;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Lancet. 2021. PMID: 33617777 Free PMC article. - Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial.
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, Dold C, Emary KRW, Fedosyuk S, Fuskova M, Gbesemete D, Green C, Hallis B, Hou MM, Jenkin D, Joe CCD, Kelly EJ, Kerridge S, Lawrie AM, Lelliott A, Lwin MN, Makinson R, Marchevsky NG, Mujadidi Y, Munro APS, Pacurar M, Plested E, Rand J, Rawlinson T, Rhead S, Robinson H, Ritchie AJ, Ross-Russell AL, Saich S, Singh N, Smith CC, Snape MD, Song R, Tarrant R, Themistocleous Y, Thomas KM, Villafana TL, Warren SC, Watson MEE, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Faust SN, Pollard AJ; Oxford COVID Vaccine Trial Group. Ramasamy MN, et al. Lancet. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. Epub 2020 Nov 19. Lancet. 2021. PMID: 33220855 Free PMC article. Clinical Trial. - Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial.
Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, Blane B, Bonsall D, Cicconi P, Charlton S, Clutterbuck EA, Collins AM, Cox T, Darton TC, Dold C, Douglas AD, Duncan CJA, Ewer KJ, Flaxman AL, Faust SN, Ferreira DM, Feng S, Finn A, Folegatti PM, Fuskova M, Galiza E, Goodman AL, Green CM, Green CA, Greenland M, Hallis B, Heath PT, Hay J, Hill HC, Jenkin D, Kerridge S, Lazarus R, Libri V, Lillie PJ, Ludden C, Marchevsky NG, Minassian AM, McGregor AC, Mujadidi YF, Phillips DJ, Plested E, Pollock KM, Robinson H, Smith A, Song R, Snape MD, Sutherland RK, Thomson EC, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Williams CJ, Hill AVS, Lambe T, Gilbert SC, Voysey M, Ramasamy MN, Pollard AJ; COVID-19 Genomics UK consortium; AMPHEUS Project; Oxford COVID-19 Vaccine Trial Group. Emary KRW, et al. Lancet. 2021 Apr 10;397(10282):1351-1362. doi: 10.1016/S0140-6736(21)00628-0. Epub 2021 Mar 30. Lancet. 2021. PMID: 33798499 Free PMC article. Clinical Trial. - Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Folegatti PM, et al. Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702298 Free PMC article. Clinical Trial. - An exploratory analysis of the response to ChAdOx1 nCoV-19 (AZD1222) vaccine in males and females.
Marchevsky NG, Li G, Aley P, Costa Clemens SA, Barrett JR, Belij-Rammerstorfer S, Bibi S, Clutterbuck E, Dold C, Felle S, Flaxman A, Folegatti P, Jenkin D, Gilbert S, Kelly S, Lambe T, Plested E, Ramasamy M, Singh N, Smith H, Taylor S, Weckx L, Pollard AJ, Voysey M; Oxford COVID Vaccine Trial Group. Marchevsky NG, et al. EBioMedicine. 2022 Jul;81:104128. doi: 10.1016/j.ebiom.2022.104128. Epub 2022 Jun 30. EBioMedicine. 2022. PMID: 35779491 Free PMC article.
Cited by
- COVID-19 vaccine uptake and associated factors among individuals living in a peri-urban area in Uganda: A cross-sectional study.
Nanteza MB, Nanyonjo G, Kyakuwa N, Nakanjako F, Kalute H, Atuhairwe C, Watera C, Ssemwanga D. Nanteza MB, et al. PLoS One. 2024 Nov 4;19(11):e0312377. doi: 10.1371/journal.pone.0312377. eCollection 2024. PLoS One. 2024. PMID: 39495801 Free PMC article. - COVID-19 Vaccination in Patients with Autoimmune Inflammatory Rheumatic Diseases: Clinical Guidance of the Korean College of Rheumatology.
Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ; Korean College of Rheumatology Task Force for COVID-19 Vaccine Guidance for Patients with Autoimmune Inflammatory Rheumatic Diseases. Park JK, et al. J Korean Med Sci. 2021 Mar 29;36(12):e95. doi: 10.3346/jkms.2021.36.e95. J Korean Med Sci. 2021. PMID: 33783147 Free PMC article. Review. - Utility of Time-Variant Multiphase CTA Color Maps in Outcome Prediction for Acute Ischemic Stroke Due to Anterior Circulation Large Vessel Occlusion.
Ospel JM, Cimflova P, Volny O, Qiu W, Hafeez M, Mayank A, Najm M, Chung K, Kashani N, Almekhlafi MA, Menon BK, Goyal M. Ospel JM, et al. Clin Neuroradiol. 2021 Sep;31(3):783-790. doi: 10.1007/s00062-020-00958-3. Epub 2020 Sep 25. Clin Neuroradiol. 2021. PMID: 32975611 - Biobehavioral Aspects of the COVID-19 Pandemic: A Review.
Hall PA, Sheeran P, Fong GT, Cheah CSL, Oremus M, Liu-Ambrose T, Sakib MN, Butt ZA, Ayaz H, Jandu N, Morita PP. Hall PA, et al. Psychosom Med. 2021 May 1;83(4):309-321. doi: 10.1097/PSY.0000000000000932. Psychosom Med. 2021. PMID: 33790201 Free PMC article. Review. - Case-Control Study of Individuals with Discrepant Nucleocapsid and Spike Protein SARS-CoV-2 IgG Results.
Wang H, Wiredja D, Yang L, Bulterys PL, Costales C, Röltgen K, Manalac J, Yee J, Zehnder J, Shi RZ, Boyd SD, Pinsky BA. Wang H, et al. Clin Chem. 2021 Jul 6;67(7):977-986. doi: 10.1093/clinchem/hvab045. Clin Chem. 2021. PMID: 33720347 Free PMC article.
References
- WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int
- WHO DRAFT landscape of COVID-19 candidate vaccines—20 March 2020. 2020. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronav...
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12014/8/MRC_/Medical Research Council/United Kingdom
- MC_PC_19055/MRC_/Medical Research Council/United Kingdom
- MR/T031891/1/MRC_/Medical Research Council/United Kingdom
- MR/L006588/1/MRC_/Medical Research Council/United Kingdom
- MR/N008219/1/MRC_/Medical Research Council/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00004/04/MRC_/Medical Research Council/United Kingdom
- MR/T001151/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_18058/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/22/MRC_/Medical Research Council/United Kingdom
- 109965/Z/15/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous