Label-free vibrational imaging of different Aβ plaque types in Alzheimer's disease reveals sequential events in plaque development - PubMed (original) (raw)
Label-free vibrational imaging of different Aβ plaque types in Alzheimer's disease reveals sequential events in plaque development
Dominik Röhr et al. Acta Neuropathol Commun. 2020.
Abstract
The neuropathology of Alzheimer's disease (AD) is characterized by hyperphosphorylated tau neurofibrillary tangles (NFTs) and amyloid-beta (Aβ) plaques. Aβ plaques are hypothesized to follow a development sequence starting with diffuse plaques, which evolve into more compact plaques and finally mature into the classic cored plaque type. A better molecular understanding of Aβ pathology is crucial, as the role of Aβ plaques in AD pathogenesis is under debate. Here, we studied the deposition and fibrillation of Aβ in different plaque types with label-free infrared and Raman imaging. Fourier-transform infrared (FTIR) and Raman imaging was performed on native snap-frozen brain tissue sections from AD cases and non-demented control cases. Subsequently, the scanned tissue was stained against Aβ and annotated for the different plaque types by an AD neuropathology expert. In total, 160 plaques (68 diffuse, 32 compact, and 60 classic cored plaques) were imaged with FTIR and the results of selected plaques were verified with Raman imaging. In diffuse plaques, we detect evidence of short antiparallel β-sheets, suggesting the presence of Aβ oligomers. Aβ fibrillation significantly increases alongside the proposed plaque development sequence. In classic cored plaques, we spatially resolve cores containing predominantly large parallel β-sheets, indicating Aβ fibrils. Combining label-free vibrational imaging and immunohistochemistry on brain tissue samples of AD and non-demented cases provides novel insight into the spatial distribution of the Aβ conformations in different plaque types. This way, we reconstruct the development process of Aβ plaques in human brain tissue, provide insight into Aβ fibrillation in the brain, and support the plaque development hypothesis.
Keywords: Alzheimer’s disease; Amyloid plaque; Amyloid-beta; FTIR; Fibril; Human; Imaging; Infrared, Raman; Microspectroscopy; Oligomer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
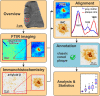
Workflow. Fourier-transform infrared (FTIR) and Raman imaging were applied to selected sample areas. Subsequently, the sample was immunostained against amyloid beta (Aβ) and imaged with light microscopy. The resulting (spectral) images were spatially aligned to generate a precisely overlaid, unified dataset. An experienced neuropathologist (B.D.C.B) annotated Aβ-IHC images to the different plaque types. Based on this data, spectral analysis was conducted and statistically evaluated
Fig. 2
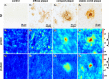
Immunohistochemical and FTIR imaging of control tissue (1) diffuse (2), compact (3) and classic cored plaques (4). A Immunohistochemical staining against amyloid beta (Aβ). B Ratio between the Amide II and CH stretching bands. Red indicates high protein concentrations. C Ration between the main β-sheet band and non-β-sheet band of the Amide I. Red indicates high β-sheet levels
Fig. 3
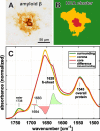
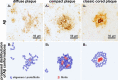
FTIR analysis of an exemplary classic cored plaque and its compartments. A Anti-Aβ immunostaining B Areas of spectral similarity, identified by hierarchical cluster analysis (HCA), that correspond to surrounding tissue (green), corona (yellow) and core (red). C Area-normalized FTIR spectra in the range 1780–1480 cm−1. The red core spectrum shows a prominent shoulder around 1628 cm−1, and reduced absorbance around 1655 cm−1. A difference spectrum (black) in the in the range 1700–1600 cm−1 reveals minor bands around 1683 cm−1 and 1694 cm−1. The lipid-associated ester band around 1738 cm−1 is decreased in both the corona and the core
Fig. 4
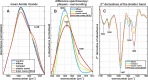
Amide I band analysis. A Mean Amide I bands of all plaque spectra from each plaque type, core spectra generated by hierarchial cluster analysis (HCA), and gray matter control spectra. The shoulder around 1628 cm−1 indicates β-sheet protein. B Cutout of mean difference spectra between plaque spectra and their respective surrounding spectra. Note the shift to lower wavenumbers and the increased absorbance around 1620 cm−1. C Visualization of sub-bands of the Amide I in the region 1700–1600 cm−1. The marked local minima indicate bands that are relevant for protein secondary structure. Note the substantial increase of the band around 1628 cm−1 alongside the plaque development sequence. The band around 1693 cm−1 displays little change, whereas the band around 1682 cm−1 increases, and the bands around 1657 cm−1, and 1639 cm−1 decrease
Fig. 5
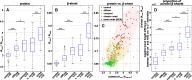
Statistical analysis. The boxplots present spectroscopic ratios derived from control, plaque, and core spectra. The red bar indicates the median value, the blue boxes range between the first and third quartile. The black whiskers extend the extremes of the distribution, excluding outliers (black crosses). The significance bars announce the confidence levels. A Ratios between the Amide II band and the CH stretching bands, indicating protein accumulation. B Ratios between the Amide I band of β-sheets and non-β-sheet structures, indicating β-sheet levels. C The scatterplot illustrates the correlation between protein and β-sheet levels in plaques. A successive accumulation of β-sheet protein alongside the plaque development sequence is apparent. D The negative height difference of the bands around 1628 and 1693 cm−1 in 2nd derivative spectra, indicating increased proportions of parallel β-sheets
Fig. 6
Proposal of Aβ conformations in the different plaque types. A depicts the exemplary plaques from Fig. 2. B Based on our observations, we propose the depicted composition of Aβ conformations in the different plaque types. The symbols are used to indicate the hypothetical location, density, and mixture of Aβ conformation in a simplified fashion
Similar articles
- Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-β plaques in Alzheimer's disease.
Ikonomovic MD, Buckley CJ, Abrahamson EE, Kofler JK, Mathis CA, Klunk WE, Farrar G. Ikonomovic MD, et al. Acta Neuropathol. 2020 Oct;140(4):463-476. doi: 10.1007/s00401-020-02175-1. Epub 2020 Aug 9. Acta Neuropathol. 2020. PMID: 32772265 Free PMC article. - Pyroglutamation of amyloid-βx-42 (Aβx-42) followed by Aβ1-40 deposition underlies plaque polymorphism in progressing Alzheimer's disease pathology.
Michno W, Nyström S, Wehrli P, Lashley T, Brinkmalm G, Guerard L, Syvänen S, Sehlin D, Kaya I, Brinet D, Nilsson KPR, Hammarström P, Blennow K, Zetterberg H, Hanrieder J. Michno W, et al. J Biol Chem. 2019 Apr 26;294(17):6719-6732. doi: 10.1074/jbc.RA118.006604. Epub 2019 Feb 27. J Biol Chem. 2019. PMID: 30814252 Free PMC article. - Virtual histology of Alzheimer's disease: Biometal entrapment within amyloid-β plaques allows for detection via X-ray phase-contrast imaging.
Chourrout M, Sandt C, Weitkamp T, Dučić T, Meyronet D, Baron T, Klohs J, Rama N, Boutin H, Singh S, Olivier C, Wiart M, Brun E, Bohic S, Chauveau F. Chourrout M, et al. Acta Biomater. 2023 Oct 15;170:260-272. doi: 10.1016/j.actbio.2023.07.046. Epub 2023 Aug 11. Acta Biomater. 2023. PMID: 37574159 - Non-canonical soluble amyloid-beta aggregates and plaque buffering: controversies and future directions for target discovery in Alzheimer's disease.
Brody DL, Jiang H, Wildburger N, Esparza TJ. Brody DL, et al. Alzheimers Res Ther. 2017 Aug 17;9(1):62. doi: 10.1186/s13195-017-0293-3. Alzheimers Res Ther. 2017. PMID: 28818091 Free PMC article. Review. - Neuritic Plaques - Gateways to Understanding Alzheimer's Disease.
Tsering W, Prokop S. Tsering W, et al. Mol Neurobiol. 2024 May;61(5):2808-2821. doi: 10.1007/s12035-023-03736-7. Epub 2023 Nov 8. Mol Neurobiol. 2024. PMID: 37940777 Free PMC article. Review.
Cited by
- Isotope Encoded chemical Imaging Identifies Amyloid Plaque Age Dependent Structural Maturation, Synaptic Loss, and Increased Toxicity.
Wood JI, Dulewicz M, Ge J, Stringer K, Szadziewska A, Desai S, Koutarapu S, Hajar HB, Blennow K, Zetterberg H, Cummings DM, Savas JN, Edwards FA, Hanrieder J. Wood JI, et al. bioRxiv [Preprint]. 2024 Oct 11:2024.10.08.617019. doi: 10.1101/2024.10.08.617019. bioRxiv. 2024. PMID: 39416086 Free PMC article. Preprint. - Infrared spectral profiling of demyelinating activity in multiple sclerosis brain tissue.
Gakh O, Wilkins JM, Guo Y, Popescu BF, Weigand SD, Kalinowska-Lyszczarz A, Lucchinetti CF. Gakh O, et al. Acta Neuropathol Commun. 2024 Sep 10;12(1):146. doi: 10.1186/s40478-024-01854-4. Acta Neuropathol Commun. 2024. PMID: 39256864 Free PMC article. - Thiophene-Based Ligands for Specific Assignment of Distinct Aβ Pathologies in Alzheimer's Disease.
Klingstedt T, Lantz L, Shirani H, Ge J, Hanrieder J, Vidal R, Ghetti B, Nilsson KPR. Klingstedt T, et al. ACS Chem Neurosci. 2024 Apr 3;15(7):1581-1595. doi: 10.1021/acschemneuro.4c00021. Epub 2024 Mar 24. ACS Chem Neurosci. 2024. PMID: 38523263 Free PMC article. - Synthetic β-sheets mimicking fibrillar and oligomeric structures for evaluation of spectral X-ray scattering technique for biomarker quantification.
Suresh K, Dahal E, Badano A. Suresh K, et al. Cell Biosci. 2024 Feb 19;14(1):26. doi: 10.1186/s13578-024-01208-6. Cell Biosci. 2024. PMID: 38374092 Free PMC article. - Exploration of macromolecular phenotype of human skeletal muscle in diabetes using infrared spectroscopy.
Zupančič B, Ugwoke CK, Abdelmonaem MEA, Alibegović A, Cvetko E, Grdadolnik J, Šerbec A, Umek N. Zupančič B, et al. Front Endocrinol (Lausanne). 2023 Dec 21;14:1308373. doi: 10.3389/fendo.2023.1308373. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38189046 Free PMC article.
References
- Benseny-Cases N, Álvarez-Marimon E, Castillo-Michel H, Cotte M, Falcon C, Cladera J. Synchrotron-based fourier transform infrared microspectroscopy (μFTIR) study on the effect of Alzheimer’s Aβ amorphous and fibrillar aggregates on PC12 cells. Anal Chem. 2018;90:2772–2779. doi: 10.1021/acs.analchem.7b04818. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical