Discovery, Function, and Therapeutic Targeting of Siglec-8 - PubMed (original) (raw)
Review
doi: 10.3390/cells10010019.
John Leung 1, Rustom Falahati 1, Jason Williams 1, Julia Schanin 1, Emily C Brock 1, Bhupinder Singh 1, Alan T Chang 1, Jeremy A O'Sullivan 2, Robert P Schleimer 2, Nenad Tomasevic 1, Christopher R Bebbington 1, Bruce S Bochner 2
Affiliations
- PMID: 33374255
- PMCID: PMC7823959
- DOI: 10.3390/cells10010019
Review
Discovery, Function, and Therapeutic Targeting of Siglec-8
Bradford A Youngblood et al. Cells. 2020.
Abstract
Siglecs (sialic acid-binding immunoglobulin-like lectins) are single-pass cell surface receptors that have inhibitory activities on immune cells. Among these, Siglec-8 is a CD33-related family member selectively expressed on human mast cells and eosinophils, and at low levels on basophils. These cells can participate in inflammatory responses by releasing mediators that attract or activate other cells, contributing to the pathogenesis of allergic and non-allergic diseases. Since its discovery in 2000, initial in vitro studies have found that the engagement of Siglec-8 with a monoclonal antibody or with selective polyvalent sialoglycan ligands induced the cell death of eosinophils and inhibited mast cell degranulation. Anti-Siglec-8 antibody administration in vivo to humanized and transgenic mice selectively expressing Siglec-8 on mouse eosinophils and mast cells confirmed the in vitro findings, and identified additional anti-inflammatory effects. AK002 (lirentelimab) is a humanized non-fucosylated IgG1 antibody against Siglec-8 in clinical development for mast cell- and eosinophil-mediated diseases. AK002 administration has safely demonstrated the inhibition of mast cell activity and the depletion of eosinophils in several phase 1 and phase 2 trials. This article reviews the discovery and functions of Siglec-8, and strategies for its therapeutic targeting for the treatment of eosinophil- and mast cell-associated diseases.
Keywords: AK002; Siglec-8; eosinophils; glycan ligands; lirentelimab; mast cells; monoclonal antibodies.
Conflict of interest statement
B.A.Y., J.L., R.F., J.W., J.S., E.C.B., B.S., A.T.C., N.T., and C.R.B. are current or former employees of and/or own stock and/or stock options from Allakos, Inc. J.A.O. has nothing to disclose. R.P.S. and B.S.B. receive remuneration for serving on the scientific advisory board of Allakos, Inc. and own stock in Allakos. B.S.B. receives publication-related royalty payments from Elsevier and UpToDate. R.P.S. and B.S.B. are co-inventors on existing Siglec-8–related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. Schleimer and Bochner are also co-founders of Allakos, Inc. which makes them subject to certain restrictions under University policy. The terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
Figures
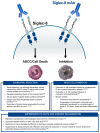
Figure 1
Summary of the in vitro and in vivo functions of Siglec-8 on eosinophils and mast cells. (Left) Eosinophil-specific activity of Siglec-8 mAbs or glycan-ligands. (Right) Mast cell-specific activity of Siglec-8 mAbs. (Bottom) Anti-inflammatory activity of targeting both eosinophils and mast cells with Siglec-8 mAbs. ADCC, antibody-dependent cellular cytotoxicity; AKC, atopic keratoconjunctivitis; CSU, chronic spontaneous urticaria; EG/EoD, eosinophilic gastritis/eosinophilic duodenitis; ISM, indolent systemic mastocytosis.
Similar articles
- AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice.
Youngblood BA, Brock EC, Leung J, Falahati R, Bryce PJ, Bright J, Williams J, Shultz LD, Greiner DL, Brehm MA, Bebbington C, Tomasevic N. Youngblood BA, et al. Int Arch Allergy Immunol. 2019;180(2):91-102. doi: 10.1159/000501637. Epub 2019 Aug 9. Int Arch Allergy Immunol. 2019. PMID: 31401630 Free PMC article. - An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells.
Kerr SC, Gonzalez JR, Schanin J, Peters MC, Lambrecht BN, Brock EC, Charbit A, Ansel KM, Youngblood BA, Fahy JV. Kerr SC, et al. Clin Exp Allergy. 2020 Aug;50(8):904-914. doi: 10.1111/cea.13681. Epub 2020 Jul 8. Clin Exp Allergy. 2020. PMID: 32542913 Free PMC article. - "Siglec"ting the allergic response for therapeutic targeting.
Bochner BS. Bochner BS. Glycobiology. 2016 Jun;26(6):546-52. doi: 10.1093/glycob/cww024. Epub 2016 Feb 23. Glycobiology. 2016. PMID: 26911285 Free PMC article. Review. - Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells.
O'Sullivan JA, Carroll DJ, Cao Y, Salicru AN, Bochner BS. O'Sullivan JA, et al. J Allergy Clin Immunol. 2018 May;141(5):1774-1785.e7. doi: 10.1016/j.jaci.2017.06.028. Epub 2017 Jul 20. J Allergy Clin Immunol. 2018. PMID: 28734845 Free PMC article. - Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions.
Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Kiwamoto T, et al. Pharmacol Ther. 2012 Sep;135(3):327-36. doi: 10.1016/j.pharmthera.2012.06.005. Epub 2012 Jun 27. Pharmacol Ther. 2012. PMID: 22749793 Free PMC article. Review.
Cited by
- Optimizing Siglec-8-Directed Immunotherapy for Eosinophilic and Mast Cell Disorders.
Lim SYT, Huo J, Laszlo GS, Cole FM, Kehret AR, Li J, Lunn-Halbert MC, Persicke JL, Rupert PB, Strong RK, Walter RB. Lim SYT, et al. Cancers (Basel). 2024 Oct 14;16(20):3476. doi: 10.3390/cancers16203476. Cancers (Basel). 2024. PMID: 39456570 Free PMC article. - Biologics in Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis.
Ezekwe E, Weskamp AL, Pittman LM, Klion AD. Ezekwe E, et al. Immunol Allergy Clin North Am. 2024 Nov;44(4):629-644. doi: 10.1016/j.iac.2024.07.003. Epub 2024 Aug 16. Immunol Allergy Clin North Am. 2024. PMID: 39389714 Review. - Sialylated keratan sulfates on MUC5B are Siglec-8 ligands in the human esophagus.
Li TA, Gonzalez-Gil A, Awol AK, Ackerman SJ, Orsburn BC, Schnaar RL. Li TA, et al. Glycobiology. 2024 Aug 30;34(10):cwae065. doi: 10.1093/glycob/cwae065. Glycobiology. 2024. PMID: 39173029 - The New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis: Biological Drugs.
Ridolo E, Barone A, Ottoni M, Peveri S, Montagni M, Nicoletta F. Ridolo E, et al. Int J Mol Sci. 2024 Jan 30;25(3):1702. doi: 10.3390/ijms25031702. Int J Mol Sci. 2024. PMID: 38338983 Free PMC article. Review. - Genetic Changes in Mastocytes and Their Significance in Mast Cell Tumor Prognosis and Treatment.
Zmorzynski S, Kimicka-Szajwaj A, Szajwaj A, Czerwik-Marcinkowska J, Wojcierowski J. Zmorzynski S, et al. Genes (Basel). 2024 Jan 22;15(1):137. doi: 10.3390/genes15010137. Genes (Basel). 2024. PMID: 38275618 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical