Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine - PubMed (original) (raw)
Clinical Trial
. 2021 Feb 4;384(5):403-416.
doi: 10.1056/NEJMoa2035389. Epub 2020 Dec 30.
Hana M El Sahly 1, Brandon Essink 1, Karen Kotloff 1, Sharon Frey 1, Rick Novak 1, David Diemert 1, Stephen A Spector 1, Nadine Rouphael 1, C Buddy Creech 1, John McGettigan 1, Shishir Khetan 1, Nathan Segall 1, Joel Solis 1, Adam Brosz 1, Carlos Fierro 1, Howard Schwartz 1, Kathleen Neuzil 1, Larry Corey 1, Peter Gilbert 1, Holly Janes 1, Dean Follmann 1, Mary Marovich 1, John Mascola 1, Laura Polakowski 1, Julie Ledgerwood 1, Barney S Graham 1, Hamilton Bennett 1, Rolando Pajon 1, Conor Knightly 1, Brett Leav 1, Weiping Deng 1, Honghong Zhou 1, Shu Han 1, Melanie Ivarsson 1, Jacqueline Miller 1, Tal Zaks 1; COVE Study Group
Collaborators, Affiliations
- PMID: 33378609
- PMCID: PMC7787219
- DOI: 10.1056/NEJMoa2035389
Clinical Trial
Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine
Lindsey R Baden et al. N Engl J Med. 2021.
Abstract
Background: Vaccines are needed to prevent coronavirus disease 2019 (Covid-19) and to protect persons who are at high risk for complications. The mRNA-1273 vaccine is a lipid nanoparticle-encapsulated mRNA-based vaccine that encodes the prefusion stabilized full-length spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes Covid-19.
Methods: This phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at 99 centers across the United States. Persons at high risk for SARS-CoV-2 infection or its complications were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (100 μg) or placebo 28 days apart. The primary end point was prevention of Covid-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with SARS-CoV-2.
Results: The trial enrolled 30,420 volunteers who were randomly assigned in a 1:1 ratio to receive either vaccine or placebo (15,210 participants in each group). More than 96% of participants received both injections, and 2.2% had evidence (serologic, virologic, or both) of SARS-CoV-2 infection at baseline. Symptomatic Covid-19 illness was confirmed in 185 participants in the placebo group (56.5 per 1000 person-years; 95% confidence interval [CI], 48.7 to 65.3) and in 11 participants in the mRNA-1273 group (3.3 per 1000 person-years; 95% CI, 1.7 to 6.0); vaccine efficacy was 94.1% (95% CI, 89.3 to 96.8%; P<0.001). Efficacy was similar across key secondary analyses, including assessment 14 days after the first dose, analyses that included participants who had evidence of SARS-CoV-2 infection at baseline, and analyses in participants 65 years of age or older. Severe Covid-19 occurred in 30 participants, with one fatality; all 30 were in the placebo group. Moderate, transient reactogenicity after vaccination occurred more frequently in the mRNA-1273 group. Serious adverse events were rare, and the incidence was similar in the two groups.
Conclusions: The mRNA-1273 vaccine showed 94.1% efficacy at preventing Covid-19 illness, including severe disease. Aside from transient local and systemic reactions, no safety concerns were identified. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; COVE ClinicalTrials.gov number, NCT04470427.).
Copyright © 2020 Massachusetts Medical Society.
Conflict of interest statement
Dr. Baden reports being funded by the NIH to conduct clinical trials in collaboration with Crucell/Janssen and Moderna; Dr. Rouphael, receiving grant support from Pfizer, Merck, Sanofi–Pasteur, Eli Lilly, and Quidel; Dr. Creech, receiving grant support from Merck, consulting fees from Horizon Pharma and GSK, and fees for serving on a data and safety monitoring board from Astellas; Dr. Neuzil, receiving grant support from Pfizer; Dr. Graham, holding pending patent WO/2018/081318 on prefusion coronavirus spike proteins and their use and pending patent 62/972,886 on 2019-nCoV vaccine; Dr. Bennett, being employed by and owning stock and stock options in Moderna; Dr. Pajon, being employed by and owning stock in Moderna; Dr. Knightly, being employed by and owning stock and stock options in Moderna; Drs. Leav, Deng, and Zhou being employees of Moderna; Dr. Han, being employed by and owning stock and stock options in Moderna; Dr. Ivarsson, being employed by and owning share options in Moderna; Dr. Miller, being employed by and owning stock and stock options in Moderna; and Dr. Zaks, being employed by and owning stock options in Moderna. No other potential conflict of interest relevant to this article was reported.
Figures
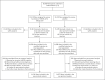
Figure 1. Randomization and Analysis Populations.
The data cutoff for the primary analysis occurred on November 25, 2020. The full analysis population consisted of participants who underwent randomization and received at least one dose of mRNA-1273 or placebo; the modified intention-to-treat population comprised participants in the full analysis population who had no immunologic or virologic evidence of Covid-19 on day 1, before the first dose; and the per-protocol analysis population included participants in the modified intention-to-treat population who received two doses, with no major protocol deviations. The safety population included all participants who received at least one injection. Among participants who received an incorrect injection, three participants in the mRNA-1273 group received at least one dose of placebo and no dose of mRNA-1273 and were included in the placebo safety population, and three received one dose of placebo and one dose of mRNA-1273 and were included in the mRNA-1273 safety population; in the placebo group all seven received mRNA-1273 and were included in the mRNA-1273 safety population. Participants who received dose 2 outside the window for the per-protocol analysis are those who did not receive the second dose between 7 days before and 14 days after day 29.
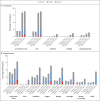
Figure 2. Solicited Local and Systemic Adverse Events.
Shown is the percentage of participants who had a solicited local or systemic adverse event within 7 days after injection 1 or injection 2 of either the placebo or the mRNA-1273 vaccine.
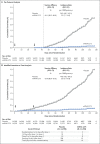
Figure 3. Vaccine Efficacy of mRNA-1273 to Prevent Covid-19.
Shown is the cumulative incidence of Covid-19 events in the primary analysis based on adjudicated assessment starting 14 days after the second vaccination in the per-protocol population (Panel A) and after randomization in the modified intention-to-treat population (Panel B) (see the Supplementary Appendix). The dotted line in Panel A indicates day 42 (14 days after vaccination 2), when the per-protocol follow-up began, and arrows in both panels indicate days 1 and 29, when injections were administered. Tick marks indicate censored data. Vaccine efficacy was defined as 1 minus the hazard ratio (mRNA vs. placebo), and the 95% confidence interval was estimated with the use of a stratified Cox proportional hazards model, with Efron’s method of tie handling and with treatment group as a covariate, with adjustment for stratification factor. Incidence was defined as the number of events divided by number of participants at risk and was adjusted by person-years. Symptomatic Covid-19 case accrual for placebo and vaccine in the modified intention-to-treat population is displayed (does not include asymptomatic cases of SARS-CoV-2 detected at the day 29 by nasopharyngeal swab).
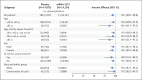
Figure 4. Vaccine Efficacy of mRNA-1273 to Prevent Covid-19 in Subgroups.
The efficacy of the RNA-1273 vaccine in preventing Covid-19 in various subgroups in the per-protocol population was based on adjudicated assessments starting 14 days after the second injection. Vaccine efficacy, defined as 1 minus the hazard ratio (mRNA-1273 vs. placebo), and 95% confidence intervals were estimated with the use of a stratified Cox proportional hazards model, with Efron’s method of tie handling and with the treatment group as a covariate, adjusting for stratification factor if applicable. Race and ethnic group categories shown are White (non-Hispanic) and communities of color (all others, including those whose race and ethnicity were both reported as unknown, were not reported, or were both missing at screening). Data for communities of color were pooled owing to limited numbers of participants in each racial or ethnic group, to ensure that the subpopulations would be large enough for meaningful analyses.
Download a PDF of the Research Summary.
Comment in
- A New Vaccine to Battle Covid-19.
Haynes BF. Haynes BF. N Engl J Med. 2021 Feb 4;384(5):470-471. doi: 10.1056/NEJMe2035557. Epub 2020 Dec 30. N Engl J Med. 2021. PMID: 33378607 Free PMC article. No abstract available. - COVID-19: Nobelpreiswürdiger Erfolg in der Impfstoff-Forschung.
Holzgreve H. Holzgreve H. MMW Fortschr Med. 2021 Feb;163(2):24-25. doi: 10.1007/s15006-021-9577-4. MMW Fortschr Med. 2021. PMID: 33527275 Free PMC article. Review. German. No abstract available. - In high-risk adults, the Moderna vaccine had 94% efficacy against COVID-19 ≥14 d after the 2nd dose.
Chagla Z. Chagla Z. Ann Intern Med. 2021 Mar;174(3):JC28. doi: 10.7326/ACPJ202103160-028. Epub 2021 Mar 2. Ann Intern Med. 2021. PMID: 33646836 - Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2.
Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, Hashimoto D, Banerji A, Li L, Anvari S, Shenoy ES. Blumenthal KG, et al. N Engl J Med. 2021 Apr 1;384(13):1273-1277. doi: 10.1056/NEJMc2102131. Epub 2021 Mar 3. N Engl J Med. 2021. PMID: 33657292 Free PMC article. No abstract available.
Similar articles
- Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase.
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, Zervos M, Rankin B, Eder F, Feldman G, Kennelly C, Han-Conrad L, Levin M, Neuzil KM, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Polakowski L, Mascola JR, Ledgerwood JE, Graham BS, August A, Clouting H, Deng W, Han S, Leav B, Manzo D, Pajon R, Schödel F, Tomassini JE, Zhou H, Miller J; COVE Study Group. El Sahly HM, et al. N Engl J Med. 2021 Nov 4;385(19):1774-1785. doi: 10.1056/NEJMoa2113017. Epub 2021 Sep 22. N Engl J Med. 2021. PMID: 34551225 Free PMC article. Clinical Trial. - Efficacy of a bivalent (D614 + B.1.351) SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant in adults: a phase 3, parallel, randomised, modified double-blind, placebo-controlled trial.
Dayan GH, Rouphael N, Walsh SR, Chen A, Grunenberg N, Allen M, Antony J, Asante KP, Bhate AS, Beresnev T, Bonaparte MI, Celle M, Ceregido MA, Corey L, Dobrianskyi D, Fu B, Grillet MH, Keshtkar-Jahromi M, Juraska M, Kee JJ, Kibuuka H, Koutsoukos M, Masotti R, Michael NL, Neuzil KM, Reynales H, Robb ML, Villagómez Martínez SM, Sawe F, Schuerman L, Tong T, Treanor J, Wartel TA, Diazgranados CA, Chicz RM, Gurunathan S, Savarino S, Sridhar S; VAT00008 Study Team. Dayan GH, et al. Lancet Respir Med. 2023 Nov;11(11):975-990. doi: 10.1016/S2213-2600(23)00263-1. Epub 2023 Sep 13. Lancet Respir Med. 2023. PMID: 37716365 Free PMC article. Clinical Trial. - Comparative efficacy and safety of COVID-19 vaccines in phase III trials: a network meta-analysis.
Wu X, Xu K, Zhan P, Liu H, Zhang F, Song Y, Lv T. Wu X, et al. BMC Infect Dis. 2024 Feb 21;24(1):234. doi: 10.1186/s12879-023-08754-3. BMC Infect Dis. 2024. PMID: 38383356 Free PMC article. Review. - The success of SARS-CoV-2 vaccines and challenges ahead.
Subbarao K. Subbarao K. Cell Host Microbe. 2021 Jul 14;29(7):1111-1123. doi: 10.1016/j.chom.2021.06.016. Cell Host Microbe. 2021. PMID: 34265245 Free PMC article. Review.
Cited by
- Safety of Simultaneous vs Sequential mRNA COVID-19 and Inactivated Influenza Vaccines: A Randomized Clinical Trial.
Walter EB, Schlaudecker EP, Talaat KR, Rountree W, Broder KR, Duffy J, Grohskopf LA, Poniewierski MS, Spreng RL, Staat MA, Tekalign R, Museru O, Goel A, Davis GN, Schmader KE. Walter EB, et al. JAMA Netw Open. 2024 Nov 4;7(11):e2443166. doi: 10.1001/jamanetworkopen.2024.43166. JAMA Netw Open. 2024. PMID: 39504023 Clinical Trial. - Nanomedicine-based strategies for the treatment of vein graft disease.
Zhou Z, Chen W, Cao Y, Abdi R, Tao W. Zhou Z, et al. Nat Rev Cardiol. 2024 Nov 5. doi: 10.1038/s41569-024-01094-y. Online ahead of print. Nat Rev Cardiol. 2024. PMID: 39501093 Review. - Exploring vaccine hesitancy in digital public discourse: From tribal polarization to socio-economic disparities.
Ayaz H, Celik MH, Koytak HZ, Yanik IE. Ayaz H, et al. PLoS One. 2024 Nov 5;19(11):e0308122. doi: 10.1371/journal.pone.0308122. eCollection 2024. PLoS One. 2024. PMID: 39499705 Free PMC article. - High-throughput synthesis and optimization of ionizable lipids through A3 coupling for efficient mRNA delivery.
Li J, Hu J, Jin D, Huo H, Chen N, Lin J, Lu X. Li J, et al. J Nanobiotechnology. 2024 Nov 4;22(1):672. doi: 10.1186/s12951-024-02919-1. J Nanobiotechnology. 2024. PMID: 39497197 Free PMC article. - COVID-19 vaccine uptake and associated factors among individuals living in a peri-urban area in Uganda: A cross-sectional study.
Nanteza MB, Nanyonjo G, Kyakuwa N, Nakanjako F, Kalute H, Atuhairwe C, Watera C, Ssemwanga D. Nanteza MB, et al. PLoS One. 2024 Nov 4;19(11):e0312377. doi: 10.1371/journal.pone.0312377. eCollection 2024. PLoS One. 2024. PMID: 39495801 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI148689/AI/NIAID NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- I01 BX003714/BX/BLRD VA/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- L40 AI154610/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- K23 AI159399/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- R01 AI134878/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- K12 HD052892/HD/NICHD NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- Contract No. 75A50120C00034/US/United States/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous