Development and Content Validation of Pruritus and Symptoms Assessment for Atopic Dermatitis (PSAAD) in Adolescents and Adults with Moderate-to-Severe AD - PubMed (original) (raw)
. 2021 Feb;11(1):221-233.
doi: 10.1007/s13555-020-00474-9. Epub 2021 Feb 3.
Mark G Lebwohl 2, Andrew G Bushmakin 3, Eric L Simpson 4, Melinda J Gooderham 5 6, Andreas Wollenberg 7, Adam Gater 8, Jane R Wells 8, Joseph C Cappelleri 9, Ming-Ann Hsu 10, Jocelyn Papacharalambous 11, Elena Peeva 12, Anna M Tallman 11, Weidong Zhang 13, Linda Chen 11
Affiliations
- PMID: 33382444
- PMCID: PMC7859139
- DOI: 10.1007/s13555-020-00474-9
Development and Content Validation of Pruritus and Symptoms Assessment for Atopic Dermatitis (PSAAD) in Adolescents and Adults with Moderate-to-Severe AD
Rebecca Hall et al. Dermatol Ther (Heidelb). 2021 Feb.
Erratum in
- Correction to: Development and Content Validation of Pruritus and Symptoms Assessment for Atopic Dermatitis (PSAAD) in Adolescents and Adults with Moderate-to-Severe AD.
Hall R, Lebwohl MG, Bushmakin AG, Simpson EL, Gooderham MJ, Wollenberg A, Gater A, Wells JR, Cappelleri JC, Hsu MA, Papacharalambous J, Peeva E, Tallman AM, Zhang W, Chen L. Hall R, et al. Dermatol Ther (Heidelb). 2021 Aug;11(4):1449-1450. doi: 10.1007/s13555-021-00567-z. Dermatol Ther (Heidelb). 2021. PMID: 34181217 Free PMC article. No abstract available.
Abstract
Introduction: Most patient-reported outcome (PRO) instruments that measure atopic dermatitis (AD) symptoms do not have sufficient documented evidence of content validity to satisfy regulatory agency guidance for inclusion in product-labelling claims in the USA or Europe. The objective of this study was to develop a PRO instrument in accordance with regulatory agency guidance to assess daily AD symptoms during the course of therapy and to establish its content validity and psychometric properties.
Methods: The Pruritus and Symptoms Assessment for Atopic Dermatitis (PSAAD) daily diary was developed based on qualitative interviews with US adolescents and adults with mild-to-severe AD. Content validity, test-retest reliability, internal consistency reliability, clinically important difference, clinically important responder, convergent validity, and known-group validity were evaluated using correlational and regression methods from phase 2b data from US adults with moderate-to-severe AD who were treated with abrocitinib.
Results: Patient interviews conducted with US adolescents and adults with mild-to-severe AD identified 11 relevant symptoms (itch, dryness, redness, flaking, discolouration, pain, bleeding, cracking, bumps, swelling, and weeping/oozing) for inclusion in the PSAAD instrument. All PSAAD psychometric parameters were acceptable based on phase 2b data from US adults with moderate-to-severe AD. Convergent validity and known-group validity were confirmed by significant correlations between PSAAD and six other PRO measures (r = 0.24-0.91, all p ≤ 0.01) and Dermatology Life Quality Index category (p ≤ 0.0001), respectively.
Conclusions: Evidence supports the PSAAD instrument validity, reliability, responsiveness and definitions of clinically important changes/differences for adults with moderate-to-severe AD.
Keywords: Atopic dermatitis; Daily diary; Eczema; Patient-reported outcomes; Pruritus.
Figures
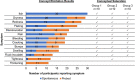
Fig. 1
Summary of concept elicitation and conceptual saturation results for atopic dermatitis symptoms. The number of spontaneous (blue) and probed (orange) reports of each symptom are displayed along with the group of concept elicitation transcripts with which each symptom was spontaneously mentioned (checkmarks) to assess conceptual saturation. Note: Interviews were divided into three equally sized groups (group 1, group 2, group 3)
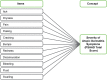
Fig. 2
PSAAD diary conceptual framework. AD atopic dermatitis, PSAAD Pruritus and Symptoms Assessment for Atopic Dermatitis
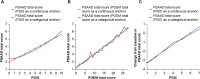
Fig. 3
Relationship between a PSAAD total score and PGIS, b PSAAD total score and POEM total score and c change from baseline in PSAAD total score and PGIC. PGIC patient global impression of change, PGIS patient global impression of severity, POEM Patient-Oriented Eczema Measure, PSAAD Pruritus and Symptoms Assessment for Atopic Dermatitis
Similar articles
- Abrocitinib Provides Rapid and Sustained Improvement in Skin Pain and Is Associated with Improved Quality of Life Outcomes in Adult and Adolescent Patients with Moderate-to-Severe Atopic Dermatitis.
Thyssen JP, Bewley A, Ständer S, Castro C, Misery L, Kim BS, Biswas P, Chan G, Myers DE, Watkins M, Alderfer J, Güler E, Silverberg JI. Thyssen JP, et al. Dermatology. 2024;240(2):243-253. doi: 10.1159/000535285. Epub 2023 Dec 11. Dermatology. 2024. PMID: 38081155 Free PMC article. Clinical Trial. - Psychometric properties of the itch numeric rating scale, skin pain numeric rating scale, and atopic dermatitis sleep scale in adult patients with moderate-to-severe atopic dermatitis.
Silverberg JI, DeLozier A, Sun L, Thyssen JP, Kim B, Yosipovitch G, Nunes FP, Gugiu PC, Doll HA, Eichenfield LF. Silverberg JI, et al. Health Qual Life Outcomes. 2021 Oct 23;19(1):247. doi: 10.1186/s12955-021-01877-8. Health Qual Life Outcomes. 2021. PMID: 34688290 Free PMC article. - Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis.
Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, Clark M, Williams N, Chen Z, Ardeleanu M, Akinlade B, Graham NMH, Pirozzi G, Staudinger H, Plaum S, Radin A, Gadkari A. Yosipovitch G, et al. Br J Dermatol. 2019 Oct;181(4):761-769. doi: 10.1111/bjd.17744. Epub 2019 May 1. Br J Dermatol. 2019. PMID: 30729499 Free PMC article. - Patient-Reported Outcome Measures in Atopic Dermatitis and Chronic Hand Eczema in Adults.
Barrett A, Hahn-Pedersen J, Kragh N, Evans E, Gnanasakthy A. Barrett A, et al. Patient. 2019 Oct;12(5):445-459. doi: 10.1007/s40271-019-00373-y. Patient. 2019. PMID: 31270775 Free PMC article. Review.
Cited by
- Abrocitinib Provides Rapid and Sustained Improvement in Skin Pain and Is Associated with Improved Quality of Life Outcomes in Adult and Adolescent Patients with Moderate-to-Severe Atopic Dermatitis.
Thyssen JP, Bewley A, Ständer S, Castro C, Misery L, Kim BS, Biswas P, Chan G, Myers DE, Watkins M, Alderfer J, Güler E, Silverberg JI. Thyssen JP, et al. Dermatology. 2024;240(2):243-253. doi: 10.1159/000535285. Epub 2023 Dec 11. Dermatology. 2024. PMID: 38081155 Free PMC article. Clinical Trial. - Efficacy and safety of abrocitinib for moderate-to-severe atopic dermatitis in adolescents and adults: Meta-analysis.
Li L, Yu J, Chen B, Guo Y, Yang Y. Li L, et al. Front Pharmacol. 2023 May 4;14:1154949. doi: 10.3389/fphar.2023.1154949. eCollection 2023. Front Pharmacol. 2023. PMID: 37214438 Free PMC article. Review. - Treatment-resistant atopic dermatitis: novel therapeutics, digital tools, and precision medicine.
Naik PP. Naik PP. Asia Pac Allergy. 2022 Apr 28;12(2):e20. doi: 10.5415/apallergy.2022.12.e20. eCollection 2022 Apr. Asia Pac Allergy. 2022. PMID: 35571547 Free PMC article. Review. - Patient-reported outcomes from the JADE COMPARE randomized phase 3 study of abrocitinib in adults with moderate-to-severe atopic dermatitis.
Thyssen JP, Yosipovitch G, Paul C, Kwatra SG, Chu CY, DiBonaventura M, Feeney C, Zhang F, Myers D, Rojo R, Valdez H. Thyssen JP, et al. J Eur Acad Dermatol Venereol. 2022 Mar;36(3):434-443. doi: 10.1111/jdv.17813. Epub 2021 Dec 13. J Eur Acad Dermatol Venereol. 2022. PMID: 34779063 Free PMC article. Clinical Trial. - Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: an analysis of patient-reported outcomes.
Cork MJ, McMichael A, Teng J, Valdez H, Rojo R, Chan G, Zhang F, Myers DE, DiBonaventura M. Cork MJ, et al. J Eur Acad Dermatol Venereol. 2022 Mar;36(3):422-433. doi: 10.1111/jdv.17792. Epub 2021 Dec 4. J Eur Acad Dermatol Venereol. 2022. PMID: 34743361 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials