Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19 - PubMed (original) (raw)
. 2021 Apr;70(4):698-706.
doi: 10.1136/gutjnl-2020-323020. Epub 2021 Jan 11.
Tao Zuo # 2 3 4, Grace Chung-Yan Lui 3 5, Fen Zhang 2 3 4, Qin Liu 2 3 4, Amy Yl Li 3, Arthur Ck Chung 2 3 4, Chun Pan Cheung 2 3 4, Eugene Yk Tso 6, Kitty Sc Fung 7, Veronica Chan 6, Lowell Ling 8, Gavin Joynt 8, David Shu-Cheong Hui 3 5, Kai Ming Chow 3, Susanna So Shan Ng 3 5, Timothy Chun-Man Li 3 5, Rita Wy Ng 1, Terry Cf Yip 3 4, Grace Lai-Hung Wong 3 4, Francis Kl Chan 2 3 4, Chun Kwok Wong # 9, Paul Ks Chan 1 2 10, Siew C Ng 11 3 4
Affiliations
- PMID: 33431578
- PMCID: PMC7804842
- DOI: 10.1136/gutjnl-2020-323020
Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19
Yun Kit Yeoh et al. Gut. 2021 Apr.
Abstract
Objective: Although COVID-19 is primarily a respiratory illness, there is mounting evidence suggesting that the GI tract is involved in this disease. We investigated whether the gut microbiome is linked to disease severity in patients with COVID-19, and whether perturbations in microbiome composition, if any, resolve with clearance of the SARS-CoV-2 virus.
Methods: In this two-hospital cohort study, we obtained blood, stool and patient records from 100 patients with laboratory-confirmed SARS-CoV-2 infection. Serial stool samples were collected from 27 of the 100 patients up to 30 days after clearance of SARS-CoV-2. Gut microbiome compositions were characterised by shotgun sequencing total DNA extracted from stools. Concentrations of inflammatory cytokines and blood markers were measured from plasma.
Results: Gut microbiome composition was significantly altered in patients with COVID-19 compared with non-COVID-19 individuals irrespective of whether patients had received medication (p<0.01). Several gut commensals with known immunomodulatory potential such as Faecalibacterium prausnitzii, Eubacterium rectale and bifidobacteria were underrepresented in patients and remained low in samples collected up to 30 days after disease resolution. Moreover, this perturbed composition exhibited stratification with disease severity concordant with elevated concentrations of inflammatory cytokines and blood markers such as C reactive protein, lactate dehydrogenase, aspartate aminotransferase and gamma-glutamyl transferase.
Conclusion: Associations between gut microbiota composition, levels of cytokines and inflammatory markers in patients with COVID-19 suggest that the gut microbiome is involved in the magnitude of COVID-19 severity possibly via modulating host immune responses. Furthermore, the gut microbiota dysbiosis after disease resolution could contribute to persistent symptoms, highlighting a need to understand how gut microorganisms are involved in inflammation and COVID-19.
Keywords: colonic bacteria; colonic microflora; inflammation.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Figure 1
Compositional differences in gut microbiota between patients with COVID-19 and non-COVID-19 subjects. (A) Average relative abundances of microbial phyla detected in stools from in-hospital patients with COVID-19, patients discharged after negative RT-qPCR for viral RNA in nasopharyngeal swabs, and non-COVID-19 individuals. (B) Principal component analysis of gut microbiota composition of patients with COVID-19 with and without antibiotics compared with non-COVID-19 subjects. Filled circles represent the first stool samples (if serial samples are available) of in-hospital patients whereas crosses represent non-COVID-19 subjects. Group centroids are indicated by the group labels.
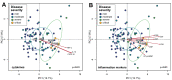
Figure 2
Associations between gut microbiota composition of in-hospital patients with COVID-19 and plasma concentrations of inflammatory cytokines and blood inflammation markers. (A) Principal component analysis (PCA) of gut microbiota composition and association with plasma concentrations of cytokines/chemokines. (B) PCA of gut microbiota composition and association with blood inflammation markers. Statistical correlations were determined using Procrustes tests. Only cytokines and inflammation markers significantly correlated with gut microbiota composition are shown. Red arrows represent gradients of the corresponding cytokines/inflammation marker concentrations and point to the direction of greatest increase in these measures. Colour of the circles represents disease severity groups, and ellipses represent SD of the group centroid. Group centroids are indicated by the group labels. AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase; NT-proBNP, N-terminal-pro-brain natriuretic peptide; TNF, tumour necrosis factor.
Figure 3
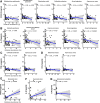
Correlations between COVID-19 enriched/depleted gut microbial taxa and plasma concentrations of (A) CXCL10, (B) IL-10, (C) TNF-α, (D) CCL2, (E) CXCL8, (F) IL-1β and (G) IL-6. Only statistically significant correlations are shown. Linear regression lines are shown in each scatter plot in blue, and shaded regions represent 95% CIs. CCL, C-C motif ligand; CXCL, C-X-C motif ligand; TNF, tumour necrosis factor.
Figure 4
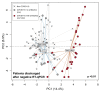
Principal component analysis of gut microbiota composition in recovered patients with COVID-19 who had or had not received antibiotics compared with non-COVID-19 subjects. Patients were considered recovered following negative quantitative reverse transcription PCR (RT-qPCR) tests for SARS-CoV-2 RNA in nasopharyngeal swabs. Filled circles represent all stools collected after discharge from hospital whereas crosses represent non-COVID-19 subjects.
Comment in
- Six-month follow-up of gut microbiota richness in patients with COVID-19.
Chen Y, Gu S, Chen Y, Lu H, Shi D, Guo J, Wu WR, Yang Y, Li Y, Xu KJ, Ding C, Luo R, Huang C, Yu L, Xu M, Yi P, Liu J, Tao JJ, Zhang H, Lv L, Wang B, Sheng J, Li L. Chen Y, et al. Gut. 2022 Jan;71(1):222-225. doi: 10.1136/gutjnl-2021-324090. Epub 2021 Apr 8. Gut. 2022. PMID: 33833065 Free PMC article. No abstract available. - Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection.
Nashed L, Mani J, Hazrati S, Stern DB, Subramanian P, Mattei L, Bittinger K, Hu W, Levy S, Maxwell GL, Hourigan SK. Nashed L, et al. Gut. 2022 Nov;71(11):2371-2373. doi: 10.1136/gutjnl-2021-326599. Epub 2022 Feb 8. Gut. 2022. PMID: 35135843 Free PMC article. No abstract available.
Similar articles
- Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization.
Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Zuo T, et al. Gastroenterology. 2020 Sep;159(3):944-955.e8. doi: 10.1053/j.gastro.2020.05.048. Epub 2020 May 20. Gastroenterology. 2020. PMID: 32442562 Free PMC article. - COVID-19 and the Human Gut Microbiome: An Under-Recognized Association.
Abbasi AF, Marinkovic A, Prakash S, Sanyaolu A, Smith S. Abbasi AF, et al. Chonnam Med J. 2022 Sep;58(3):96-101. doi: 10.4068/cmj.2022.58.3.96. Epub 2022 Sep 23. Chonnam Med J. 2022. PMID: 36245770 Free PMC article. Review. - SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19?
Chattopadhyay I, Shankar EM. Chattopadhyay I, et al. Front Cell Infect Microbiol. 2021 Mar 11;11:590874. doi: 10.3389/fcimb.2021.590874. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791231 Free PMC article. - Gut microbiome and anti-viral immunity in COVID-19.
Rossini V, Tolosa-Enguis V, Frances-Cuesta C, Sanz Y. Rossini V, et al. Crit Rev Food Sci Nutr. 2024;64(14):4587-4602. doi: 10.1080/10408398.2022.2143476. Epub 2022 Nov 16. Crit Rev Food Sci Nutr. 2024. PMID: 36382631 Review.
Cited by
- Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia.
Bernard-Raichon L, Venzon M, Klein J, Axelrad JE, Zhang C, Sullivan AP, Hussey GA, Casanovas-Massana A, Noval MG, Valero-Jimenez AM, Gago J, Putzel G, Pironti A, Wilder E; Yale IMPACT Research Team; Thorpe LE, Littman DR, Dittmann M, Stapleford KA, Shopsin B, Torres VJ, Ko AI, Iwasaki A, Cadwell K, Schluter J. Bernard-Raichon L, et al. Nat Commun. 2022 Nov 1;13(1):5926. doi: 10.1038/s41467-022-33395-6. Nat Commun. 2022. PMID: 36319618 Free PMC article. - Impaired tryptophan metabolism in the gastrointestinal tract of patients with critical coronavirus disease 2019.
Yokoyama Y, Ichiki T, Yamakawa T, Tsuji Y, Kuronuma K, Takahashi S, Narimatsu E, Nakase H. Yokoyama Y, et al. Front Med (Lausanne). 2022 Aug 10;9:941422. doi: 10.3389/fmed.2022.941422. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36035409 Free PMC article. - Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome.
Sukocheva OA, Maksoud R, Beeraka NM, Madhunapantula SV, Sinelnikov M, Nikolenko VN, Neganova ME, Klochkov SG, Amjad Kamal M, Staines DR, Marshall-Gradisnik S. Sukocheva OA, et al. J Adv Res. 2022 Sep;40:179-196. doi: 10.1016/j.jare.2021.11.013. Epub 2021 Nov 26. J Adv Res. 2022. PMID: 36100326 Free PMC article. Review. - Is butyrate a natural alternative to dexamethasone in the management of CoVID-19?
K NK, Patil P, Bhandary SK, Haridas V, N SK, E S, Shetty P. K NK, et al. F1000Res. 2021 Apr 6;10:273. doi: 10.12688/f1000research.51786.1. eCollection 2021. F1000Res. 2021. PMID: 34046165 Free PMC article. Review. - Immunomodulatory fecal metabolites are associated with mortality in COVID-19 patients with respiratory failure.
Stutz MR, Dylla NP, Pearson SD, Lecompte-Osorio P, Nayak R, Khalid M, Adler E, Boissiere J, Lin H, Leiter W, Little J, Rose A, Moran D, Mullowney MW, Wolfe KS, Lehmann C, Odenwald M, De La Cruz M, Giurcanu M, Pohlman AS, Hall JB, Chaubard JL, Sundararajan A, Sidebottom A, Kress JP, Pamer EG, Patel BK. Stutz MR, et al. Nat Commun. 2022 Nov 3;13(1):6615. doi: 10.1038/s41467-022-34260-2. Nat Commun. 2022. PMID: 36329015 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous