Reconfiguration of metabolic fluxes in Pseudomonas putida as a response to sub-lethal oxidative stress - PubMed (original) (raw)
Reconfiguration of metabolic fluxes in Pseudomonas putida as a response to sub-lethal oxidative stress
Pablo I Nikel et al. ISME J. 2021 Jun.
Abstract
As a frequent inhabitant of sites polluted with toxic chemicals, the soil bacterium and plant-root colonizer Pseudomonas putida can tolerate high levels of endogenous and exogenous oxidative stress. Yet, the ultimate reason of such phenotypic property remains largely unknown. To shed light on this question, metabolic network-wide routes for NADPH generation-the metabolic currency that fuels redox-stress quenching mechanisms-were inspected when P. putida KT2440 was challenged with a sub-lethal H2O2 dose as a proxy of oxidative conditions. 13C-tracer experiments, metabolomics, and flux analysis, together with the assessment of physiological parameters and measurement of enzymatic activities, revealed a substantial flux reconfiguration in oxidative environments. In particular, periplasmic glucose processing was rerouted to cytoplasmic oxidation, and the cyclic operation of the pentose phosphate pathway led to significant NADPH-forming fluxes, exceeding biosynthetic demands by ~50%. The resulting NADPH surplus, in turn, fueled the glutathione system for H2O2 reduction. These properties not only account for the tolerance of P. putida to environmental insults-some of which end up in the formation of reactive oxygen species-but they also highlight the value of this bacterial host as a platform for environmental bioremediation and metabolic engineering.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
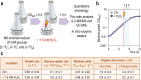
Fig. 1. Experimental overview and quantitative physiology parameters for P. putida KT2440 cultures.
a Overview of the experimental design adopted in this study. All growth and quantitative physiology experiments were conducted at least in biological triplicates, and the oxidative challenge is indicated with a red symbol. OD600, optical density measured at 600 nm. b Representative growth curves for control (Ctrl.) experiments and H2O2-stressed cultures. Samples were taken 1.5 h after addition of H2O2, where the growth of the cells was still exponential. c Key physiological parameters of P. putida KT2440 in batch glucose cultures. All values represent the mean ± standard deviations from at least three biological replicates, and the differences between the growth parameters in H2O2-treated and control cultures was not significant (as assessed by means of the Student’s t test with Welch’s correction). The specific growth rate (μ) and the specific rate of carbon uptake (_q_S) were determined during exponential growth (including the treatment period) by linear regression of log-transformed OD600 data. The yield of biomass on substrate (_Y_X/S) was calculated at 24 h, after glucose was completely exhausted. The extracellular concentration (concn.) of organic acids reported is the maximal reached during the whole culture period. CDW, cell dry weight.
Fig. 2. Metabolite levels in P. putida KT2440 under oxidative stress.
a Relative abundance of selected metabolites, grouped according to the biochemical block they belong to (i.e. PP pathway, EMP pathway, and TCA cycle). Relative metabolite abundance is expressed as the ratio between H2O2-induced oxidative stress and control (Ctrl.) conditions, derived from summed ion abundance of all isotopes (counts). Data from experiments in the presence of [1-13C1]- and [6-13C1]-glucose (Glc.) are averaged. Bars represent the mean value of metabolite abundance ± standard deviations obtained in triplicate measurements of samples from three independent experiments per labeled substrate. Statistical comparisons between the metabolite abundance ratios (with a ratio = 1 indicating no difference between stressed cultures and control experiments) were assessed by the Student’s t test with Welch’s correction. Single (*) and double asterisks (**) identify significant differences at the p < 0.05 and p < 0.01 levels, respectively. Actual p values for the metabolite ratios in the PP pathway (stressed versus control experiments) were p = 0.0052, 0.0031, 0.0092, 0.0043, and 0.0055, indicated in the same order as the bar graph. For the metabolites in the EMP pathway (stressed versus control experiments), the values were p = 0.0198, 0.0083, 0.0079, and 0.0412. For the metabolites in the TCA cycle (stressed versus control experiments), the values were p = 0.0322, 0.0049, 0.0931, and 0.0074. b Changes in selected metabolic flux ratios in upper metabolism upon exposure of the cells to H2O2. The 13C-labeled substrate used in each experiment is indicated. Bars represent averages from three independent experiments, and standard deviations were calculated using the covariance matrices of the respective mass distribution vectors by applying the Gaussian law of error propagation. c Relative pathway contribution to the G6P and F6P pools. The input of each of the metabolic pathways to the sugar phosphate pool under oxidative stress conditions is indicated with different colors. All abbreviations used in this figure are as indicated in the legend to Fig. S1. CDW, cell dry weight.
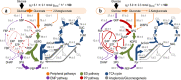
Fig. 3. In vivo carbon flux distribution in glucose-grown P. putida KT2440 obtained from ratio-constrained flux balance analysis.
All fluxes, calculated under control conditions (a) and in the presence of H2O2-induced oxidative stress (b), were normalized to the specific glucose uptake rate (_q_S), and the width of each arrow is scaled to the relative flux. Flux values represent the mean ± standard deviations from at least three biological replicates, after integration of physiological and metabolomics data together with flux ratio analysis. Dashed lines indicate that no significant flux through the corresponding biochemical step was detected under the conditions tested. Abbreviations used for the metabolic intermediates and the main metabolic blocks within the biochemical network are given in the legend to Fig. S1. CDW, cell dry weight.
Fig. 4. Dynamic NADPH balance in P. putida KT2440 upon oxidative stress.
Overall NADPH balances and sensitivity analysis for the wild-type strain under control (a, b, e, f) or H2O2-induced oxidative stress conditions (c, d, g, h) using cofactor specificities of major dehydrogenases under saturating conditions (left column) or quasi in vivo conditions (right column). NADPH formation was determined from carbon fluxes through redox cofactor-dependent reactions (Fig. 3 and Table S1) multiplied by experimentally determined relative cofactor specificities. NADPH consumption was calculated from the requirements for biomass production and the actual NADPH-dependent KguD activity. The overall rates of NADPH formation (green) and NADPH consumption (red) are individually indicated (a–d). Dependence of the overall rates of NADPH turnover on the relative GnuK/KguD flux ratio and the cofactor specificity of Zwf derived from saturating conditions or quasi in vivo conditions (e–h). Actual values for the net NADPH balance are given for the experimentally determined NADP+ specificity of Zwf (0.937) as a function of the relative GnuK/KguD flux ratio, with the calculated net rate for each condition indicated with a red dot. CDW, cell dry weight.
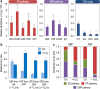
Fig. 5. In vitro analysis of key enzymatic activities and glutathione metabolism.
a Enzyme activity ratios were calculated from the specific activity for each of the indicated reactions assessed under H2O2-induced oxidative stress and control (Ctrl.) conditions. Each bar represents the mean value of the corresponding ratio ± standard deviations of triplicate measurements from at least two independent experiments, and the horizontal dashed line indicates an activity ratio = 1 (i.e. no changes in enzymatic activities across experimental conditions). Statistical comparisons between enzyme activity ratios were assessed by the Student’s t test with Welch’s correction. Single (*) and double asterisks (**) identify significant differences at the p < 0.05 and p < 0.01 levels, respectively. Actual p values for the Glk and Gad activity ratios in the glucose conversion routes were p = 0.0019 and 0.0293, respectively. Actual p values for the Zwf and GntZ activity ratios in the PP pathway were p = 0.0087 and 0.0096, respectively. Circled numbers identify the enzymes in the biochemical network of Fig. S1. b Glutathione metabolism in P. putida KT2440. The key activities involved in biosynthesis and recycling of the reduced (GSH) and oxidized (GS–SG) forms of glutathione are indicated along with the corresponding PP identifiers. c Enzymatic determination of total glutathione and the fraction of the oxidized and reduced form. Bars represent the mean value of the corresponding parameter ± standard deviations of duplicate measurements from at least five independent experiments, with individual measurements indicated as empty circles, and the asterisk (*) identifies significant differences between stressed cells and control conditions at P < 0.05 as assessed by the Student’s t test with the Bonferroni correc_t_ion. Actual p values for the total glutathione content and the GSH/GS–SG ratio between H2O2-treated and control cultures were p = 0.0361 and 0.0117, respectively. CDW, cell dry weight. d Impact of the carbon substrate on the growth of P. putida KT2440 upon an oxidative challenge. Normalized growth coefficients, representing the fraction of the specific growth rate (μ) in the presence of 3 mM H2O2 as compared with that of control (Ctrl.) conditions, were calculated in cultures using glucose, α-ketoglutarate (α-KG) or glycerol as the carbon source. Each bar represents the mean value of the normalized growth coefficients ± standard deviations of triplicate independent experiments, while the arrows and the accompanying percentages indicate the relative reduction in the growth rate under oxidative stress.
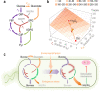
Fig. 6. The architecture of central carbon metabolism in P. putida enables rapid supply of NADPH upon oxidative stress.
a Schematic representation of the upper metabolism of P. putida KT2440. Several biochemical reaction have been lumped to illustrate the main routes for carbon circulation (see Fig. S1 for details and abbreviations). Note that the total rate of carbon uptake (_q_S) is split between glucose phosphorylation and oxidation to gluconate, such that _q_S = r p + r ox. The overall cycling flux of trioses phosphate towards hexoses phosphate is indicated as r c and the flux through the PP pathway shunt is termed r s. Cofactors other than NADPH have been omitted in the drawing for the sake of clarity. b Functional relationship between _r_NADPH, the rate of NADPH formation within the simplified metabolic network of (a), and the fluxes through the oxidative loop for glucose processing and the PP pathway shunt. All (arbitrary) values are given as a fraction of _q_S, and the experimental conditions tested in this work are indicated with red dots (Ctrl. control conditions). c General model for flux distribution in the upper metabolic domain of P. putida. Under normal growth conditions, glucose is processed mostly through its oxidative conversion to gluconate, and the EDEMP cycle provides intermediates for biomass, with a very low flux through the PP pathway. Upon oxidative stress conditions (exerted either by endogenous or external perturbations), a rapid increase of fluxes via the PP pathway shunt replenishes the intermediates within upper metabolism and provides a direct source of NADPH that can be coupled to anti-oxidant defense mechanisms against reactive oxygen species (ROS). Fluxes predominant under each condition are highlighted.
Similar articles
- Pseudomonas putida KT2440 Strain Metabolizes Glucose through a Cycle Formed by Enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and Pentose Phosphate Pathways.
Nikel PI, Chavarría M, Fuhrer T, Sauer U, de Lorenzo V. Nikel PI, et al. J Biol Chem. 2015 Oct 23;290(43):25920-32. doi: 10.1074/jbc.M115.687749. Epub 2015 Sep 8. J Biol Chem. 2015. PMID: 26350459 Free PMC article. - The Metabolic Redox Regime of Pseudomonas putida Tunes Its Evolvability toward Novel Xenobiotic Substrates.
Akkaya Ö, Pérez-Pantoja DR, Calles B, Nikel PI, de Lorenzo V. Akkaya Ö, et al. mBio. 2018 Aug 28;9(4):e01512-18. doi: 10.1128/mBio.01512-18. mBio. 2018. PMID: 30154264 Free PMC article. - Pseudomonas putida as a functional chassis for industrial biocatalysis: From native biochemistry to trans-metabolism.
Nikel PI, de Lorenzo V. Nikel PI, et al. Metab Eng. 2018 Nov;50:142-155. doi: 10.1016/j.ymben.2018.05.005. Epub 2018 May 16. Metab Eng. 2018. PMID: 29758287 Review. - GC-MS-based 13C metabolic flux analysis resolves the parallel and cyclic glucose metabolism of Pseudomonas putida KT2440 and Pseudomonas aeruginosa PAO1.
Kohlstedt M, Wittmann C. Kohlstedt M, et al. Metab Eng. 2019 Jul;54:35-53. doi: 10.1016/j.ymben.2019.01.008. Epub 2019 Mar 1. Metab Eng. 2019. PMID: 30831266 - Pseudomonas putida as a synthetic biology chassis and a metabolic engineering platform.
Martínez-García E, de Lorenzo V. Martínez-García E, et al. Curr Opin Biotechnol. 2024 Feb;85:103025. doi: 10.1016/j.copbio.2023.103025. Epub 2023 Dec 7. Curr Opin Biotechnol. 2024. PMID: 38061264 Review.
Cited by
- Diversity pattern and antibiotic activity of microbial communities inhabiting a karst cave from Costa Rica.
Vásquez-Castro F, Wicki-Emmenegger D, Fuentes-Schweizer P, Nassar-Míguez L, Rojas-Gätjens D, Rojas-Jimenez K, Chavarría M. Vásquez-Castro F, et al. Microbiology (Reading). 2024 Nov;170(11):001513. doi: 10.1099/mic.0.001513. Microbiology (Reading). 2024. PMID: 39530301 Free PMC article. - Enhanced biosynthesis of poly(3-hydroxybutyrate) in engineered strains of Pseudomonas putida via increased malonyl-CoA availability.
Favoino G, Krink N, Schwanemann T, Wierckx N, Nikel PI. Favoino G, et al. Microb Biotechnol. 2024 Nov;17(11):e70044. doi: 10.1111/1751-7915.70044. Microb Biotechnol. 2024. PMID: 39503721 Free PMC article. - A versatile microbial platform as a tunable whole-cell chemical sensor.
Hernández-Sancho JM, Boudigou A, Alván-Vargas MVG, Freund D, Arnling Bååth J, Westh P, Jensen K, Noda-García L, Volke DC, Nikel PI. Hernández-Sancho JM, et al. Nat Commun. 2024 Sep 27;15(1):8316. doi: 10.1038/s41467-024-52755-y. Nat Commun. 2024. PMID: 39333077 Free PMC article. - Elucidating the Role of O2 Uncoupling for the Adaptation of Bacterial Biodegradation Reactions Catalyzed by Rieske Oxygenases.
Bopp CE, Bernet NM, Meyer F, Khan R, Robinson SL, Kohler HE, Buller R, Hofstetter TB. Bopp CE, et al. ACS Environ Au. 2024 May 14;4(4):204-218. doi: 10.1021/acsenvironau.4c00016. eCollection 2024 Jul 17. ACS Environ Au. 2024. PMID: 39035869 Free PMC article. - Pseudomonas putida KT2440: the long journey of a soil-dweller to become a synthetic biology chassis.
de Lorenzo V, Pérez-Pantoja D, Nikel PI. de Lorenzo V, et al. J Bacteriol. 2024 Jul 25;206(7):e0013624. doi: 10.1128/jb.00136-24. Epub 2024 Jul 8. J Bacteriol. 2024. PMID: 38975763 Review.
References
- Belda E, van Heck RGA, López-Sánchez MJ, Cruveiller S, Barbe V, Fraser C, et al. The revisited genome of Pseudomonas putida KT2440 enlightens its value as a robust metabolic chassis. Environ Microbiol. 2016;18:3403–24. - PubMed
- Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VAP, et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol. 2002;4:799–808. - PubMed
- Volke DC, Calero P, Nikel PI. Pseudomonas putida. Trends Microbiol. 2020;28:512–3. - PubMed
- Nikel PI, Chavarría M, Danchin A, de Lorenzo V. From dirt to industrial applications: Pseudomonas putida as a synthetic biology chassis for hosting harsh biochemical reactions. Curr Opin Chem Biol. 2016;34:20–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials