Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering - PubMed (original) (raw)
Review
Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering
Fei Teng et al. Adv Sci (Weinh). 2020.
Abstract
Extracellular vesicles (EVs) are biocompatible, nano-sized secreted vesicles containing many types of biomolecules, including proteins, RNAs, DNAs, lipids, and metabolites. Their low immunogenicity and ability to functionally modify recipient cells by transferring diverse bioactive constituents make them an excellent candidate for a next-generation drug delivery system. Here, the recent advances in EV biology and emerging strategies of EV bioengineering are summarized, and the prospects for clinical translation of bioengineered EVs and the challenges to be overcome are discussed.
Keywords: bioengineering; delivery systems; exosomes; extracellular vesicles; microvesicles.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
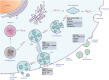
Figure 1
Biogenesis and secretion of EVs. Two mechanisms of EV biogenesis are illustrated. The process of releasing exosomes into the extracellular milieu contains three distinct steps: exosome biogenesis, intracellular trafficking of MVBs, and fusion of MVBs with the plasma membrane. Early endosomes are formed by the inward budding of the plasma membrane (Step 1a1), or in some cases from the _trans_‐Golgi network (TGN) (Step 1a2). Early endosomes mature into late endosomes (Step 1b) and finally generate MVBs, in which process ILVs are formed by inward invagination of the endosome limiting membrane (Step 2). The fate of MVBs can be fusion with the plasma membrane (Step 3a1), which results in the release of exosomes (Step 3a2). Alternatively, MVBs can fuse with lysosomes/autophagosomes for degradation (Step 3b1, 2). Several molecules are involved in the biogenesis (e.g., RABs, ESCRTs, syndecan, ceramide, tetraspanins, etc.), trafficking (e.g., RABs, actin, etc.), and fusion of MVBs with the plasma membrane (e.g., SNAREs). Microvesicles arise from the direct outward budding and fission of the plasma membrane. Several molecules are involved in the biogenesis and release of microvesicles (small GTPases, ESCRTs, ARRDC1, etc.). Abbreviations: EV, extracellular vesicle; ESCRT, endosome sorting complex required for transport; MVB, multivesicular body; ILV, intraluminal vesicle; RAB, RAS‐related protein; ALIX, ALG‐2 interacting protein X; nSMase2, neutral sphingomyelinase 2; Ral‐1, RAL (Ras‐related GTPase) homolog; SNARE, soluble NSF attachment protein receptor; VAMP7, vesicle‐associated membrane protein 7; SNAP23, synaptosomal‐associated protein 23; Syx1A, syntaxin 1A; ARF, ADP ribosylation factor; RohA, Ras homolog family member A; A‐SMase, acid sphingomyelinase; ARRDC1, arrestin domain containing protein 1. *, homologs in C. elegans.
Figure 2
MVB biogenesis machineries. Multiple molecular mechanisms of ILV generation in MVB have been revealed. A) In the canonical ESCRT‐dependent pathway, ubiquitinated proteins in the endosomal membrane are recognized by ESCRT‐0, which is recruited to the endosomal membrane by PtdIns3P binding and subsequently clustered into microdomains via clathrin binding. Then ESCRT‐0 recruits ESCRT‐I, and ESCRT‐I recruits ESCRT‐II. ESCRT‐I and ESCRT‐II coordinately induce the budding of the endosomal membrane and confine cargos within the buds. ESCRT‐III components are dynamically recruited for membrane scission of the ILV necks and disassembled after ILV scission via VPS4. In a non‐canonical ESCRT‐dependent pathway, HD‐PTP binds to ESCRT‐0 and coordinately recruits ESCRT‐I and ESCRT‐III, bypassing the need for ESCRT‐II. B) In the syndecan‐syntenin‐ALIX pathway, membrane budding and cargo clustering can occur independently of ubiquitin and ESCRT‐0, but ESCRT‐III and VPS4 are required for the scission step. C) Ceramide, generated from sphingomyelin by mSMase2, plays a key role in the ESCRT‐independent pathway of ILV biogenesis. Ceramide can form lipid raft microdomains, which might trigger the conversion of ILVs into MVBs. D) CD63 plays a vital role in the ESCRT‐independent pathway of ILV biogenesis. CD63 can form tetraspanin‐enriched microdomains, which might trigger the conversion of ILVs into MVBs. Abbreviations: MVB, multivesicular body; ILV, intraluminal vesicle; ESCRT, endosome sorting complex required for transport; PdtIns3P, phosphatidylinositol 3‐phosphate; STAM, signal transducing adaptor molecule; HRS, hepatocyte growth factor‐regulated tyrosine kinase substrate; TSG101, tumor susceptibility gene 101 protein; VPS, vacuolar protein sorting; MVB12, multivesicular body subunit 12; CHMP, charged multivesicular body protein; HD‐PTP, His domain protein tyrosine phosphatase; ALIX, ALG‐2 interacting protein X; nSMase2, neutral sphingomyelinase 2.
Figure 3
Uptake and fate of EVs. EVs can trigger intracellular signaling of recipient cells via ligand‐receptor interaction, such as antigen presentation, immune modulation and morphogen signaling. Alternatively, exogenous EVs can transfer their cargos into recipient cells by entering the cells. EVs can be internalized into recipient cells by different mechanisms, including membrane fusion and various endocytic pathways (e.g., receptor‐mediated endocytosis, caveolin‐mediated endocytosis, lipid raft‐mediated endocytosis, phagocytosis and macropinocytosis). The fusion of EVs with the plasma membrane can release their contents into the cytoplasm of recipient cells, while the endocytosed EVs reach MVBs via the canonical endosomal pathway. These internalized EVs might be degraded after the fusion of MVBs with lysosomes or be secreted from recipient cells mixed with endogenous ILVs (not shown). Also, they might back‐fuse with the limiting membrane of MVBs, leading to the release of the EV cargos into the cytoplasm, a process that is poorly understood. Abbreviations: EV, extracellular vesicle; MVB, multivesicular body; ILV, intraluminal vesicle; TCR, T‐cell receptor; MHC, major histocompatibility complex; PD1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; Fz, Frizzled receptor.
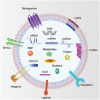
Figure 4
EV composition. EV contains various DNAs, RNAs, proteins, lipids, and metabolites. Some of these components are involved in the biogenesis of EVs, whereas most others are enriched in EVs during biogenesis. DNAs include dsDNA, ssDNA, and mtDNA. RNAs include mRNA, miRNA, lncRNA, tRNA, snoRNA, snRNA, Y‐RNA, vRNA, piRNA, mtRNA, and circRNA. Membrane proteins include tetraspanins (e.g., CD63, CD9, and CD81), MHC molecules, integrins, ligands (e.g., Hh, PD‐L1), receptors (e.g., EGFR, PDGFR), and flotillins. Lumen proteins include TSG101, ALIX, HSPs, syntenin, and RAB GTPases. Lipids include ceramide, phosphatidylserine, sphingomyelin, and cholesterol. Note that no single EV contains all of these components. Abbreviations: EV, extracellular vesicle; dsDNA, double‐stranded DNA, ssDNA, single‐stranded DNA; mtDNA, mitochondrial DNA; mRNA, messenger RNA; miRNA, microRNA; lncRNA, long non‐coding RNA; tRNA, transfer RNA; snoRNA, small nucleolar RNA; snRNA, small nuclear RNA; vRNA, vault RNA; piRNA, piwi‐interacting RNA; mtRNA, mitochondrial RNA; circRNA, circular RNA; MHC, major histocompatibility complex; Hh, Hedgehog; PD‐L1, programmed death‐ligand 1; EGFR, epidermal growth factor receptor; PDGFR, platelet‐derived growth factor receptor; TSG101, tumor susceptibility gene 101 protein; ALIX, ALG‐2 interacting protein X; HSP, heat shock protein; RBP, RNA binding protein.
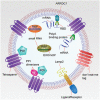
Figure 5
EV bioengineering. Various therapeutic nucleic acids and proteins can be incorporated into EVs through genetic engineering. Theoretically, any EV membrane protein can be used for fusion to therapeutic target moieties. To load therapeutic mRNAs into EVs, RBDs (e.g., L7Ae peptide, and Tat peptide) can be fused with EV membrane proteins (e.g., tetraspanins: CD9, CD63 or CD81, and ARRDC1), which can recruit mRNAs with their 3′UTR fused with an RBD recognition element (e.g., C/Dbox RNA structure, and TAR element). Another strategy is to fuse a poly(A) binding protein with EV membrane proteins to selectively recruit mRNAs into EVs. To load therapeutic small RNAs (e.g., miRNA, piRNA) into EVs, RBPs (e.g., Ago, PIWI‐like protein) can be fused with EV membrane proteins to recruit them into EVs. Also, EXOmotifs can be incorporated in these small RNAs to facilitate their enrichment into EV through EV‐enriched RBPs (e.g., hnRNPA2B1, and SYNCRIP). LncRNAs can be enriched into EVs by using similar strategies. To concentrate therapeutic proteins into the lumen of EVs, a pair of PPI dimerizers can be fused with EV membrane proteins and POIs, respectively (e.g., light‐induced CRY2 and CIBN, rapamycin‐induced FRB and FKBP, and leucine zipper), through which POIs can be induced to interact with EV membrane fusion proteins by inducers. Alternatively, POIs can be directly fused to EV membrane proteins. To change the tropism of EVs, different ligands or receptors (e.g., RVG, iRGD, and EGFR nanobody) can be fused with EV surface proteins (e.g., Lamp2, PDGFR, and GPI‐anchor peptide). To protect EVs from phagocytosis, don't‐eat‐me tags (e.g., CD47, CD55, CD59, and CD200) can be expressed on EVs. Abbreviations: EV, extracellular vesicle; mRNA, messenger RNA; RBD, RNA‐binding domain; Tat, transactivator of transcription; UTR, untranslated region; ARRDC1, arrestin domain‐containing protein 1; TAR, _trans_‐activating response; miRNA, microRNA, piRNA, piwi‐interacting RNA; RBP, RNA‐binding protein; Ago, Argonaute; lncRNA, long non‐coding RNA; PPI, protein‐protein interacting; POI, protein of interest; CRY2, cryptochrome 2; CIBN, truncated version of cryptochrome‐interacting basic helix–loop–helix 1; FRB, FKBP rapamycin binding; FKBP, FK506‐binding protein; EGFR, epidermal growth factor receptor; GPI, glycosylphosphatidylinositol; PDGFR, platelet‐derived growth factor receptor.
Similar articles
- Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source.
Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Meng W, et al. Drug Deliv. 2020 Dec;27(1):585-598. doi: 10.1080/10717544.2020.1748758. Drug Deliv. 2020. PMID: 32264719 Free PMC article. Review. - RNA and Protein Delivery by Cell-Secreted and Bioengineered Extracellular Vesicles.
Wang BZ, Luo LJ, Vunjak-Novakovic G. Wang BZ, et al. Adv Healthc Mater. 2022 Mar;11(5):e2101557. doi: 10.1002/adhm.202101557. Epub 2021 Nov 11. Adv Healthc Mater. 2022. PMID: 34706168 Free PMC article. Review. - Tetraspanins in extracellular vesicle formation and function.
Andreu Z, Yáñez-Mó M. Andreu Z, et al. Front Immunol. 2014 Sep 16;5:442. doi: 10.3389/fimmu.2014.00442. eCollection 2014. Front Immunol. 2014. PMID: 25278937 Free PMC article. Review. - Inhibition of extracellular vesicle biogenesis in tumor cells: A possible way to reduce tumorigenesis.
Rezaie J, Akbari A, Rahbarghazi R. Rezaie J, et al. Cell Biochem Funct. 2022 Apr;40(3):248-262. doi: 10.1002/cbf.3695. Epub 2022 Mar 14. Cell Biochem Funct. 2022. PMID: 35285964 Review. - Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics.
Yu J, Sane S, Kim JE, Yun S, Kim HJ, Jo KB, Wright JP, Khoshdoozmasouleh N, Lee K, Oh HT, Thiel K, Parvin A, Williams X, Hannon C, Lee H, Kim DK. Yu J, et al. Front Mol Biosci. 2024 Jan 3;10:1330400. doi: 10.3389/fmolb.2023.1330400. eCollection 2023. Front Mol Biosci. 2024. PMID: 38234582 Free PMC article. Review.
Cited by
- Distinct immunomodulation elicited by young versus aged extracellular vesicles in bone marrow-derived macrophages.
Livkisa D, Lee TL, Yeh WT, Jaimes MSV, Szomolay B, Liao CT, Lundy DJ. Livkisa D, et al. Immun Ageing. 2024 Oct 21;21(1):72. doi: 10.1186/s12979-024-00472-x. Immun Ageing. 2024. PMID: 39434100 Free PMC article. - Tumor-derived systems as novel biomedical tools-turning the enemy into an ally.
Desai N, Katare P, Makwana V, Salave S, Vora LK, Giri J. Desai N, et al. Biomater Res. 2023 Nov 9;27(1):113. doi: 10.1186/s40824-023-00445-z. Biomater Res. 2023. PMID: 37946275 Free PMC article. Review. - The role and applications of extracellular vesicles in osteoporosis.
Fang F, Yang J, Wang J, Li T, Wang E, Zhang D, Liu X, Zhou C. Fang F, et al. Bone Res. 2024 Jan 23;12(1):4. doi: 10.1038/s41413-023-00313-5. Bone Res. 2024. PMID: 38263267 Free PMC article. Review. - Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment.
Zhang Y, Liu X, Zeng L, Zhao X, Chen Q, Pan Y, Bai Y, Shao C, Zhang J. Zhang Y, et al. Br J Cancer. 2022 Nov;127(10):1760-1772. doi: 10.1038/s41416-022-01956-7. Epub 2022 Sep 1. Br J Cancer. 2022. PMID: 36050447 Free PMC article. - Extracellular Vesicles in Veterinary Medicine.
Moccia V, Sammarco A, Cavicchioli L, Castagnaro M, Bongiovanni L, Zappulli V. Moccia V, et al. Animals (Basel). 2022 Oct 10;12(19):2716. doi: 10.3390/ani12192716. Animals (Basel). 2022. PMID: 36230457 Free PMC article. Review.
References
- Yáñez‐Mó M., Siljander P. R.‐M., Andreu Z., Bedina Zavec A., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro‐Da Silva A., Fais S., Falcon‐Perez J. M., Ghobrial I. M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N. H. H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj‐Iglic V., Krämer‐Albers E.‐M., Laitinen S., Lässer C., Lener T., Ligeti E., Linē A., Lipps G., Llorente A., Lötvall J., Manček‐Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte‐’T Hoen E. N. M., Nyman T. A., O'Driscoll L., Olivan M., Oliveira C., Pállinger É., Del Portillo H. A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez‐Madrid F., Santarém N., Schallmoser K., Stampe Ostenfeld M., Stoorvogel W., Stukelj R., Van Der Grein S. G., Helena Vasconcelos M., Wauben M. H. M., De Wever O., J. Extracell. Vesicles 2015, 4, 27066. - PMC - PubMed
- Théry C., Ostrowski M., Segura E., Nat. Rev. Immunol. 2009, 9, 581. - PubMed
- Andaloussi S. E. L., Mäger I., Breakefield X. O., Wood M. J. A., Nat. Rev. Drug Discovery 2013, 12, 347. - PubMed
- Thompson A. G., Gray E., Heman‐Ackah S. M., Mäger I., Talbot K., Andaloussi S. E.l, Wood M. J., Turner M. R., Nat. Rev. Neurol. 2016, 12, 346. - PubMed
- Van Niel G., D'angelo G., Raposo G., Nat. Rev. Mol. Cell Biol. 2018, 19, 213. - PubMed
Publication types
LinkOut - more resources
Full Text Sources