Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2021 Jan 19;325(3):254-264.
doi: 10.1001/jama.2020.25864.
Renato D Lopes 1 2 3 4, Ariane V S Macedo 1 5 6, Renata J Moll-Bernardes 1, Tiago M Dos Santos 3 7, Lilian Mazza 3, André Feldman 1 8, Guilherme D'Andréa Saba Arruda 1 9, Denílson C de Albuquerque 1 10, Angelina S Camiletti 1 4, Andréa S de Sousa 1 11 12, Thiago C de Paula 5, Karla G D Giusti 13, Rafael A M Domiciano 8, Márcia M Noya-Rabelo 14 15, Alan M Hamilton 14, Vitor A Loures 8, Rodrigo M Dionísio 16, Thyago A B Furquim 16, Fábio A De Luca 17, Ítalo B Dos Santos Sousa 17, Bruno S Bandeira 18, Cleverson N Zukowski 19, Ricardo G G de Oliveira 20, Noara B Ribeiro 21, Jeffer L de Moraes 22, João L F Petriz 23, Adriana M Pimentel 24, Jacqueline S Miranda 25, Bárbara E de Jesus Abufaiad 26, C Michael Gibson 27, Christopher B Granger 2, John H Alexander 2, Olga F de Souza 1 4 25; BRACE CORONA Investigators
Affiliations
- PMID: 33464336
- PMCID: PMC7816106
- DOI: 10.1001/jama.2020.25864
Randomized Controlled Trial
Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial
Renato D Lopes et al. JAMA. 2021.
Abstract
Importance: It is unknown whether angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) have a positive, neutral, or negative effect on clinical outcomes in patients with coronavirus disease 2019 (COVID-19).
Objective: To determine whether discontinuation compared with continuation of ACEIs or ARBs changed the number of days alive and out of the hospital through 30 days.
Design, setting, and participants: A randomized clinical trial of 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization (enrolled: April 9-June 26, 2020; final follow-up: July 26, 2020).
Interventions: Discontinuation (n = 334) or continuation (n = 325) of ACEIs or ARBs.
Main outcomes and measures: The primary outcome was the number of days alive and out of the hospital through 30 days. Secondary outcomes included death, cardiovascular death, and COVID-19 progression.
Results: Among 659 patients, the median age was 55.1 years (interquartile range [IQR], 46.1-65.0 years), 14.7% were aged 70 years or older, 40.4% were women, and 100% completed the trial. The median time from symptom onset to hospital admission was 6 days (IQR, 4-9 days) and 27.2% of patients had an oxygen saturation of less than 94% of room air at baseline. In terms of clinical severity, 57.1% of patients were considered mild at hospital admission and 42.9% were considered moderate. There was no significant difference in the number of days alive and out of the hospital in patients in the discontinuation group (mean, 21.9 days [SD, 8 days]) vs patients in the continuation group (mean, 22.9 days [SD, 7.1 days]) and the mean ratio was 0.95 (95% CI, 0.90-1.01). There also was no statistically significant difference in death (2.7% for the discontinuation group vs 2.8% for the continuation group; odds ratio [OR], 0.97 [95% CI, 0.38-2.52]), cardiovascular death (0.6% vs 0.3%, respectively; OR, 1.95 [95% CI, 0.19-42.12]), or COVID-19 progression (38.3% vs 32.3%; OR, 1.30 [95% CI, 0.95-1.80]). The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs 7.7% in the continuation group), shock requiring vasopressors (8.4% vs 7.1%, respectively), acute myocardial infarction (7.5% vs 4.6%), new or worsening heart failure (4.2% vs 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs 2.8%).
Conclusions and relevance: Among patients hospitalized with mild to moderate COVID-19 and who were taking ACEIs or ARBs before hospital admission, there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue vs continue these medications. These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT04364893.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Lopes reported receiving grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Sanofi, and Pfizer; and receiving consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Sanofi, and Portola. Dr Macedo reported receiving consulting fees from Pfizer, Bayer, AstraZeneca, Novartis, Daiichi-Sankyo, Zodiac, Roche, and Janssen. Dr de Barros E Silva reported receiving grants from Pfizer, Bayer, and Roche Diagnostics; and receiving consulting fees from Pfizer, Bayer, and Roche Diagnostics. Dr Feldman reported receiving consulting fees from Pfizer, Bayer, Daiichi-Sankyo, Boehringer, and Servier. Dr D’Andrea Saba Arruda reported receiving consulting fees from Bayer, Pfizer, Servier, AstraZeneca, and Daichii Sankyo. Dr de Albuquerque reported receiving consulting fees from Boehringer Ingelheim, AstraZeneca, Bayer, and Servier. Dr de Oliveira reported receiving consulting fees from Bayer, Boehringer-Ingelheim, Servier, and Novartis. Dr Petriz reported receiving consulting fees from Bayer, Pfizer, and Daichii Sankyo. Dr Gibson reported receiving consulting fees and grants from Johnson & Johnson, Janssen, and Bayer. Dr Granger reported receiving grant support from AstraZeneca, the US Food and Drug Administration, the National Institutes of Health, GlaxoSmithKline, Medtronic, Novartis, Apple, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and Janssen; receiving consulting fees from AstraZeneca, Espero, GlaxoSmithKline, Medtronic, Novartis, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Merck, Roche, Eli Lilly, and Janssen; and that all relationships with industry are listed at dcri.org/about-us/conflict-of-interest/. Dr Alexander reported receiving grant support from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, GlaxoSmithKline, Ferring, the US Food and Drug Administration, the National Institutes of Health, and XaTek; and receiving consulting fees from AbbVie, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, CryoLife, CSL Behring, Novo Nordisk, Pfizer, Portola, Quantum Genomics, the US Department of Veterans Affairs, XaTek, Inositec, and Zafgen. Dr de Souza reported receiving grant support from Boehringer Ingelheim; and receiving consulting fees from Pfizer, Bayer, Daiichi-Sankyo, and Boehringer Ingelheim. No other disclosures were reported.
Figures
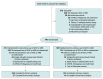
Figure 1.. Study Enrollment and Analysis in the BRACE CORONA Trial on the Use of Angiotensin-Converting Enzyme Inhibitors (ACEIs) or Angiotensin II Receptor Blockers (ARBs) Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19)
aMay have met more than 1 exclusion criterion.
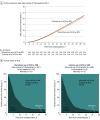
Figure 2.. Primary Outcome and Clinical Status at 30 Days
For the primary outcome, the mean number of days alive and out of the hospital was 21.9 days (SD, 8.0 days) in the discontinue use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) group vs 22.9 days (SD, 7.1 days) in the continue use of ACEI or ARB group (median, 25.0 days [interquartile range, 20.0-27.0 days] vs 25.0 days [interquartile range, 21.0-27.0 days], respectively) and the between-group mean ratio was 0.95 (95% CI, 0.90-1.01; P = .09).
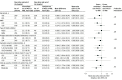
Figure 3.. Subgroup Analysis for the Primary Outcome
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CT, computed tomographic; RAAS, renin-angiotensin-aldosterone system. aThe subgroup analyses were performed using the same generalized additive model of location, scale, and shape with beta binomial distribution inflated at zero that was used for the primary outcome, including interaction terms between each subgroup and study treatments. bCalculated as weight in kilograms divided by height in meters squared. cEstimated by visual assessment performed by a radiologist. dDefined as change in clinical severity status during hospitalization. Mild defined as blood oxygen saturation of 94% or greater and lung infiltrates less than or equal to 50%; moderate, blood oxygen saturation less than 94%, or lung infiltrates greater than 50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen less than 300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Comment in
- Brugada syndrome and reduced right ventricular outflow tract conduction reserve: a final common pathway?
Behr ER, Ben-Haim Y, Ackerman MJ, Krahn AD, Wilde AAM. Behr ER, et al. Eur Heart J. 2021 Mar 14;42(11):1073-1081. doi: 10.1093/eurheartj/ehaa1051. Eur Heart J. 2021. PMID: 33421051 - A randomized trial supports the recommendation to continue treatment with ACEi or ARBs during hospitalization for COVID-19.
Volpe M, Patrono C. Volpe M, et al. Eur Heart J. 2021 Mar 14;42(11):1061-1062. doi: 10.1093/eurheartj/ehab106. Eur Heart J. 2021. PMID: 33659979 Free PMC article. No abstract available. - From confusion to clarity: RAS blockade in patients hospitalized with COVID-19.
Geherty RA, Sparks MA. Geherty RA, et al. Kidney Int. 2021 May;99(5):1059-1061. doi: 10.1016/j.kint.2021.02.034. Epub 2021 Mar 19. Kidney Int. 2021. PMID: 33753072 Free PMC article. No abstract available.
Similar articles
- Discontinuing vs continuing ACEIs and ARBs in hospitalized patients with COVID-19 according to disease severity: Insights from the BRACE CORONA trial.
Macedo AVS, de Barros E Silva PGM, de Paula TC, Moll-Bernardes RJ, Mendonça Dos Santos T, Mazza L, Feldman A, Arruda GDAS, de Albuquerque DC, de Sousa AS, de Souza OF, Gibson CM, Granger CB, Alexander JH, Lopes RD. Macedo AVS, et al. Am Heart J. 2022 Jul;249:86-97. doi: 10.1016/j.ahj.2022.04.001. Epub 2022 Apr 8. Am Heart J. 2022. PMID: 35405099 Free PMC article. Clinical Trial. - Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial.
Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, Rodriguez-Mori JE, Renna N, Chang TI, Corrales-Medina V, Andrade-Villanueva JF, Barbagelata A, Cristodulo-Cortez R, Díaz-Cucho OA, Spaak J, Alfonso CE, Valdivia-Vega R, Villavicencio-Carranza M, Ayala-García RJ, Castro-Callirgos CA, González-Hernández LA, Bernales-Salas EF, Coacalla-Guerra JC, Salinas-Herrera CD, Nicolosi L, Basconcel M, Byrd JB, Sharkoski T, Bendezú-Huasasquiche LE, Chittams J, Edmonston DL, Vasquez CR, Chirinos JA. Cohen JB, et al. Lancet Respir Med. 2021 Mar;9(3):275-284. doi: 10.1016/S2213-2600(20)30558-0. Epub 2021 Jan 7. Lancet Respir Med. 2021. PMID: 33422263 Free PMC article. Clinical Trial. - Impact of in-hospital discontinuation with angiotensin receptor blockers or converting enzyme inhibitors on mortality of COVID-19 patients: a retrospective cohort study.
de Abajo FJ, Rodríguez-Miguel A, Rodríguez-Martín S, Lerma V, García-Lledó A; MED-ACE2-COVID19 Study Group. de Abajo FJ, et al. BMC Med. 2021 May 12;19(1):118. doi: 10.1186/s12916-021-01992-9. BMC Med. 2021. PMID: 33980231 Free PMC article. - Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults.
Zou Z, Yuan HB, Yang B, Xu F, Chen XY, Liu GJ, Shi XY. Zou Z, et al. Cochrane Database Syst Rev. 2016 Jan 27;2016(1):CD009210. doi: 10.1002/14651858.CD009210.pub2. Cochrane Database Syst Rev. 2016. PMID: 26816003 Free PMC article. Review. - Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction.
Driscoll A, Currey J, Tonkin A, Krum H. Driscoll A, et al. Cochrane Database Syst Rev. 2015 Dec 21;2015(12):CD009889. doi: 10.1002/14651858.CD009889.pub2. Cochrane Database Syst Rev. 2015. PMID: 26689943 Free PMC article. Review.
Cited by
- A Comprehensive Review on Potential In Silico Screened Herbal Bioactive Compounds and Host Targets in the Cardiovascular Disease Therapy.
Zarenezhad E, Hadi AT, Nournia E, Rostamnia S, Ghasemian A. Zarenezhad E, et al. Biomed Res Int. 2024 Oct 29;2024:2023620. doi: 10.1155/2024/2023620. eCollection 2024. Biomed Res Int. 2024. PMID: 39502274 Free PMC article. Review. - Effects of Losartan on Patients Hospitalized for Acute COVID-19: A Randomized Controlled Trial.
Tran KC, Asfar P, Cheng M, Demiselle J, Singer J, Lee T, Sweet D, Boyd J, Walley K, Haljan G, Sharif O, Geri G, Auchabie J, Quenot JP, Lee TC, Tsang J, Meziani F, Lamontagne F, Dubee V, Lasocki S, Ovakim D, Wood G, Turgeon A, Cohen Y, Lebas E, Goudelin M, Forrest D, Teale A, Mira JP, Fowler R, Daneman N, Adhikari NKJ, Gousseff M, Leroy P, Plantefeve G, Rispal P, Courtois R, Winston B, Reynolds S, Birks P, Bienvenu B, Tadie JM, Talarmin JP, Ansart S, Russell JA; ARBs CORONA II Team. Tran KC, et al. Clin Infect Dis. 2024 Sep 26;79(3):615-625. doi: 10.1093/cid/ciae306. Clin Infect Dis. 2024. PMID: 39325643 Free PMC article. Clinical Trial. - Cardiovascular abnormalities of long-COVID syndrome: Pathogenic basis and potential strategy for treatment and rehabilitation.
Wu K, Van Name J, Xi L. Wu K, et al. Sports Med Health Sci. 2024 Mar 24;6(3):221-231. doi: 10.1016/j.smhs.2024.03.009. eCollection 2024 Sep. Sports Med Health Sci. 2024. PMID: 39234483 Free PMC article. Review. - Angiotensin-(1-7) infusion in COVID-19 patients admitted to the ICU: a seamless phase 1-2 randomized clinical trial.
Martins ALV, Annoni F, da Silva FA, Bolais-Ramos L, de Oliveira GC, Ribeiro RC, Diniz MML, Silva TGF, Pinheiro BD, Rodrigues NA, Dos Santos Matos AH, Motta-Santos D, Campagnole-Santos MJ, Verano-Braga T, Taccone FS, Santos RAS. Martins ALV, et al. Ann Intensive Care. 2024 Sep 4;14(1):139. doi: 10.1186/s13613-024-01369-0. Ann Intensive Care. 2024. PMID: 39231898 Free PMC article. - Guideline-directed medical therapy prescribing patterns and in-hospital outcomes among heart failure patients during COVID-19.
Srivastava PK, Klomhaus AM, Rafique A, Desai PS, Daniels LB, Yancy CW, Yang EH, Fonarow GC, Parikh RV. Srivastava PK, et al. Am Heart J Plus. 2024 Aug 2;45:100440. doi: 10.1016/j.ahjo.2024.100440. eCollection 2024 Sep. Am Heart J Plus. 2024. PMID: 39220717 Free PMC article.
References
- Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. JAMA. 2020;323(18):1769-1770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous