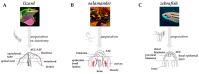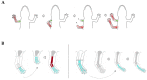Appendage Regeneration in Vertebrates: What Makes This Possible? - PubMed (original) (raw)
Review
Appendage Regeneration in Vertebrates: What Makes This Possible?
Valentina Daponte et al. Cells. 2021.
Abstract
The ability to regenerate amputated or injured tissues and organs is a fascinating property shared by several invertebrates and, interestingly, some vertebrates. The mechanism of evolutionary loss of regeneration in mammals is not understood, yet from the biomedical and clinical point of view, it would be very beneficial to be able, at least partially, to restore that capability. The current availability of new experimental tools, facilitating the comparative study of models with high regenerative ability, provides a powerful instrument to unveil what is needed for a successful regeneration. The present review provides an updated overview of multiple aspects of appendage regeneration in three vertebrates: lizard, salamander, and zebrafish. The deep investigation of this process points to common mechanisms, including the relevance of Wnt/β-catenin and FGF signaling for the restoration of a functional appendage. We discuss the formation and cellular origin of the blastema and the identification of epigenetic and cellular changes and molecular pathways shared by vertebrates capable of regeneration. Understanding the similarities, being aware of the differences of the processes, during lizard, salamander, and zebrafish regeneration can provide a useful guide for supporting effective regenerative strategies in mammals.
Keywords: FGF; WNT/β catenin; appendage regeneration; dedifferentiation; differentiation; lizard; salamander; signaling pathways; stem cells; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the content of the review.
Figures
Figure 1
Phylogenetic distribution of appendage regeneration among vertebrates according to the current literature. The blue line indicates lineages containing one or more species capable of appendage regeneration, the orange line those incapables of regeneration, and the black line those in which appendage regeneration has not yet been documented. The dashed yellow line indicates the uncertain presence of appendage regeneration in chondrichthyes and cyclostomes. The arrow indicates the reactivation of the regenerative process in amniotes.
Figure 2
Timeline activation of wound healing, blastema formation and regenerative outgrowth during appendage regeneration in vertebrates. Arrows indicate the duration of each regenerative step in lizard, salamander, and zebrafish. Hpa: hours post-amputation; dpa: days post-amputation.
Figure 3
Comparative anatomy of regenerating appendages in lizard, salamander, and zebrafish. (A) Amputation or autotomy of lizard tail induces the formation of a cone-like shaped blastema, covered by a thick wound epidermis that will form the apical epidermal peg(AEP), whose correspondence to the apical epidermal cap (AEC) found in amphibians and fish is still uncertain. During blastema formation, likely in response to AEP signals, the central canal of the original spinal cord, the ependymal tube, elongates and infiltrates the proliferating tail blastema. (B) After amputation of salamander limb, the wound epidermis quickly covers the stump. Within days, the wound epithelium becomes innervated, thickens, and becomes a specialized AEC. The AEC induces dedifferentiation in the underlying stump tissue and attracts cells, which accumulate below the AEC to form the blastema. Modified from Payzin-Dogru and Whited, 2018. (C) After zebrafish fin amputation, epithelial cells migrate to cover the wound, forming the AEC. Under the AEC, stump tissues dedifferentiate to form the blastema. Within 24 h, blastemal cells segregate into two compartments: the distal blastema, populated by slowly proliferating cells, and the proximal blastema, in which cells rapidly proliferate and differentiate to replace the amputated tissue. Dotted lines indicate the site of amputation/autotomy.
Figure 4
Experiments illustrating the principles of positional memory in salamander limb. (A) Rule of distal transformation. After the creation of a circularized limb, successive amputation induces the creation of two stumps, one with correct polarity and one with reversed polarity. Both stumps regenerate distal elements from the level of amputation, thus duplicating the distal segments already present in the reversed stump. The proximo-distal axis is indicated with a gradient of green (indicating proximal elements) and red (indicating distal elements). (B) Intercalation. Intercalary regeneration (dark red) occurs if a hand is grafted to an upper arm (left), but not if an upper arm segment is grafted to a forearm (right). Adapted from Carlson 2007.
Figure 5
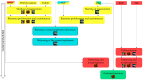
Schematic representation of the molecular signaling regulating the steps of appendage regeneration in vertebrates.
Similar articles
- New regulators of vertebrate appendage regeneration.
Yin VP, Poss KD. Yin VP, et al. Curr Opin Genet Dev. 2008 Aug;18(4):381-6. doi: 10.1016/j.gde.2008.06.008. Epub 2008 Aug 11. Curr Opin Genet Dev. 2008. PMID: 18644447 Free PMC article. Review. - Limb blastema cell: a stem cell for morphological regeneration.
Tamura K, Ohgo S, Yokoyama H. Tamura K, et al. Dev Growth Differ. 2010 Jan;52(1):89-99. doi: 10.1111/j.1440-169X.2009.01144.x. Epub 2009 Nov 5. Dev Growth Differ. 2010. PMID: 19891640 Review. - [Regeneration of vertebrate appendage: an old experimental model to study stem cells in the adult].
Tawk M, Vriz S. Tawk M, et al. Med Sci (Paris). 2003 Apr;19(4):465-71. doi: 10.1051/medsci/2003194465. Med Sci (Paris). 2003. PMID: 12836220 French. - Loss of the ability to regenerate body appendages in vertebrates: from side effects of evolutionary innovations to gene loss.
Zaraisky AG, Araslanova KR, Shitikov AD, Tereshina MB. Zaraisky AG, et al. Biol Rev Camb Philos Soc. 2024 Oct;99(5):1868-1888. doi: 10.1111/brv.13102. Epub 2024 May 30. Biol Rev Camb Philos Soc. 2024. PMID: 38817123 Review. - The influence of fundamental traits on mechanisms controlling appendage regeneration.
Seifert AW, Monaghan JR, Smith MD, Pasch B, Stier AC, Michonneau F, Maden M. Seifert AW, et al. Biol Rev Camb Philos Soc. 2012 May;87(2):330-45. doi: 10.1111/j.1469-185X.2011.00199.x. Epub 2011 Sep 19. Biol Rev Camb Philos Soc. 2012. PMID: 21929739 Review.
Cited by
- Sall4 regulates downstream patterning genes during limb regeneration.
Erickson JR, Walker SE, Arenas Gomez CM, Echeverri K. Erickson JR, et al. Dev Biol. 2024 Nov;515:151-159. doi: 10.1016/j.ydbio.2024.07.015. Epub 2024 Jul 25. Dev Biol. 2024. PMID: 39067503 - Distinct stem-like cell populations facilitate functional regeneration of the Cladonema medusa tentacle.
Fujita S, Takahashi M, Kumano G, Kuranaga E, Miura M, Nakajima YI. Fujita S, et al. PLoS Biol. 2023 Dec 21;21(12):e3002435. doi: 10.1371/journal.pbio.3002435. eCollection 2023 Dec. PLoS Biol. 2023. PMID: 38127832 Free PMC article. - New developments in the biology of fibroblast growth factors.
Ornitz DM, Itoh N. Ornitz DM, et al. WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review. - Regeneration of amputated mice digit tips by including Wnt signaling pathway with CHIR99021 and IWP-2 chemicals in limb organ culture system.
Taghiyar L, Bijarchan F, Doraj M, Baghban Eslaminejad M. Taghiyar L, et al. Iran J Basic Med Sci. 2024;27(10):1251-1259. doi: 10.22038/ijbms.2024.76957.16643. Iran J Basic Med Sci. 2024. PMID: 39229572 Free PMC article. - Spatial transcriptomics reveals asymmetric cellular responses to injury in the regenerating spiny mouse (Acomys) ear.
van Beijnum H, Koopmans T, Tomasso A, Disela V, Te Lindert S, Bakkers J, Alemany A, Berezikov E, Bartscherer K. van Beijnum H, et al. Genome Res. 2023 Aug;33(8):1424-1437. doi: 10.1101/gr.277538.122. Epub 2023 Sep 19. Genome Res. 2023. PMID: 37726147 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources