Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity - PubMed (original) (raw)
Review
Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity
Filippo Veglia et al. Nat Rev Immunol. 2021 Aug.
Abstract
Myeloid-derived suppressor cells (MDSCs) are pathologically activated neutrophils and monocytes with potent immunosuppressive activity. They are implicated in the regulation of immune responses in many pathological conditions and are closely associated with poor clinical outcomes in cancer. Recent studies have indicated key distinctions between MDSCs and classical neutrophils and monocytes, and, in this Review, we discuss new data on the major genomic and metabolic characteristics of MDSCs. We explain how these characteristics shape MDSC function and could facilitate therapeutic targeting of these cells, particularly in cancer and in autoimmune diseases. Additionally, we briefly discuss emerging data on MDSC involvement in pregnancy, neonatal biology and COVID-19.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
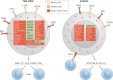
Fig. 1. Distinguishing MDSCs from classical neutrophils and monocytes.
The figure depicts the genes (depicted inside the cell) and surface molecules that can be used to distinguish polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) from classical neutrophils and monocytes. Factors are depicted in yellow, green and red based on whether they are useful markers in mice only, humans only, or both mice and humans, respectively. The figure also illustrates the main immunosuppressive mechanism used by MDSCs. Please note that this information was obtained from studies of MDSCs in cancer. CXCR1, CXC-chemokine receptor 1; FATP2, fatty acid transport protein 2; LOX1, lectin-type oxidized LDL receptor 1; NO, nitric oxide; PGE2, prostaglandin E2.
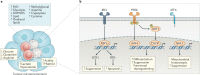
Fig. 2. Metabolic characteristics of MDSCs.
a | Changes in lipid and glucose metabolism that occur in myeloid-derived suppressor cells (MDSCs) and in tumour cells in the tumour environment are shown. MDSCs in the tumour microenvironment show an upregulation of fatty acid oxidation (FAO) and glycolysis and a decrease in oxidative phosphorylation (OXPHOS). They also show increased lipid accumulation and increased production of the metabolites methylglyoxal, arginine, tryptophan and cysteine. Key changes in the tumour microenvironment are depicted in the yellow boxes. b | Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in MDSCs. The UPR is characterized by an orchestrated upregulation of activating transcription factor 6 (ATF6), inositol requiring enzyme 1 (IRE1α) and PKR-like endoplasmic reticulum kinase (PERK). The transcription factor C/EBP-homologous protein (CHOP) is a critical mediator of the PERK pathway, whereas spliced X-box binding protein 1 (sXBP1) is a mediator of the IRE1α pathway. ER stress induced the expression of TNF-related apoptosis-induced ligand receptors (DR5) and lectin-type oxidized LDL receptor 1 (LOX1) and the conversion of neutrophils to polymorphonuclear MDSCs. The reduced NRF2 signalling favoured the accumulation of cytosolic mitochondrial DNA and consequent expression of antitumour type I interferon, in a STING-dependent manner.
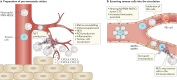
Fig. 3. Contribution of MDSCs to the formation of the premetastatic niche.
Myeloid-derived suppressor cells (MDSCs) promote metastasis by ‘priming’ the premetastatic niche to enhance the engraftment by circulating tumour cells (CTCs) (panel a) and by escorting tumour cells into the circulation (panel b), promoting their metastatic potential, inhibiting their killing by immune cells and by promoting their extravasation into the tissues. NET, neutrophil extracellular trap; NK cell, natural killer cell; MMP, matrix metalloproteinase; PMN, polymorphonuclear; ROS, reactive oxygen species.
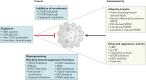
Fig. 4. Targeting MDSCs in cancer and autoimmune diseases.
Opposite strategies are used to regulate myeloid-derived suppressor cell (MDSC) function in cancer, where therapies are aimed at reducing MDSC recruitment, accumulation and suppressive functions, or in autoimmune disease, where increased MDSC accumulation or function may be used to restrain disease severity. ATRA, all-trans retinoic acid; CBD, cannabidiol; CIA, collagen-induced arthritis; CXCR, CXC-chemokine receptor; ESS, experimental Sjögren syndrome; EZH2, enhancer of zeste homologue 2; FATP2, fatty acid transporter protein 2; G-CSF, granulocyte colony-stimulating factor; LXR, liver X receptor; MPO, myeloperoxidase; NO, nitric oxide; PERK, PKR-like endoplasmic reticulum kinase; STAT3, signal transducer and activator of transcription 3.
Similar articles
- Roles of Myeloid-Derived Suppressor Cell Subpopulations in Autoimmune Arthritis.
Li M, Zhu D, Wang T, Xia X, Tian J, Wang S. Li M, et al. Front Immunol. 2018 Dec 4;9:2849. doi: 10.3389/fimmu.2018.02849. eCollection 2018. Front Immunol. 2018. PMID: 30564242 Free PMC article. Review. - Plasticity of myeloid-derived suppressor cells in cancer.
Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Tcyganov E, et al. Curr Opin Immunol. 2018 Apr;51:76-82. doi: 10.1016/j.coi.2018.03.009. Epub 2018 Mar 14. Curr Opin Immunol. 2018. PMID: 29547768 Free PMC article. Review. - New Discovery of Myeloid-Derived Suppressor Cell's Tale on Viral Infection and COVID-19.
Park SJ, Nam DE, Seong HC, Hahn YS. Park SJ, et al. Front Immunol. 2022 Feb 3;13:842535. doi: 10.3389/fimmu.2022.842535. eCollection 2022. Front Immunol. 2022. PMID: 35185933 Free PMC article. Review. - Interactions among myeloid regulatory cells in cancer.
Umansky V, Adema GJ, Baran J, Brandau S, Van Ginderachter JA, Hu X, Jablonska J, Mojsilovic S, Papadaki HA, Pico de Coaña Y, Santegoets KCM, Santibanez JF, Serre K, Si Y, Sieminska I, Velegraki M, Fridlender ZG. Umansky V, et al. Cancer Immunol Immunother. 2019 Apr;68(4):645-660. doi: 10.1007/s00262-018-2200-6. Epub 2018 Jul 12. Cancer Immunol Immunother. 2019. PMID: 30003321 Free PMC article. Review. - On the origin of myeloid-derived suppressor cells.
Millrud CR, Bergenfelz C, Leandersson K. Millrud CR, et al. Oncotarget. 2017 Jan 10;8(2):3649-3665. doi: 10.18632/oncotarget.12278. Oncotarget. 2017. PMID: 27690299 Free PMC article. Review.
Cited by
- Single cell and spatial transcriptomics highlight the interaction of club-like cells with immunosuppressive myeloid cells in prostate cancer.
Kiviaho A, Eerola SK, Kallio HML, Andersen MK, Hoikka M, Tiihonen AM, Salonen I, Spotbeen X, Giesen A, Parker CTA, Taavitsainen S, Hantula O, Marttinen M, Hermelo I, Ismail M, Midtbust E, Wess M, Devlies W, Sharma A, Krossa S, Häkkinen T, Afyounian E, Vandereyken K, Kint S, Kesseli J, Tolonen T, Tammela TLJ, Viset T, Størkersen Ø, Giskeødegård GF, Rye MB, Murtola T, Erickson A, Latonen L, Bova GS, Mills IG, Joniau S, Swinnen JV, Voet T, Mirtti T, Attard G, Claessens F, Visakorpi T, Rautajoki KJ, Tessem MB, Urbanucci A, Nykter M. Kiviaho A, et al. Nat Commun. 2024 Nov 16;15(1):9949. doi: 10.1038/s41467-024-54364-1. Nat Commun. 2024. PMID: 39550375 Free PMC article. - CXCL8 secreted by immature granulocytes inhibits WT hematopoiesis in chronic myelomonocytic leukemia.
Deschamps P, Wacheux M, Gosseye A, Morabito M, Pagès A, Lyne AM, Alfaro A, Rameau P, Imanci A, Chelbi R, Marchand V, Renneville A, Patnaik MM, Lapierre V, Badaoui B, Wagner-Ballon O, Berthon C, Braun T, Willekens C, Itzykson R, Fenaux P, Thépot S, Etienne G, Elvira-Matelot E, Porteu F, Droin N, Perié L, Laplane L, Solary E, Selimoglu-Buet D. Deschamps P, et al. J Clin Invest. 2024 Sep 17;134(22):e180738. doi: 10.1172/JCI180738. J Clin Invest. 2024. PMID: 39545419 Free PMC article. - Rg3-lipo biomimetic delivery of paclitaxel enhances targeting of tumors and myeloid-derived suppressor cells.
Shen Y, Zhong B, Zheng W, Wang D, Chen L, Song H, Pan X, Mo S, Jin B, Cui H, Zhan H, Luo F, Liu J. Shen Y, et al. J Clin Invest. 2024 Nov 15;134(22):e178617. doi: 10.1172/JCI178617. J Clin Invest. 2024. PMID: 39545407 Free PMC article. - Mitochondrial regulation in the tumor microenvironment: targeting mitochondria for immunotherapy.
Ahn M, Ali A, Seo JH. Ahn M, et al. Front Immunol. 2024 Oct 11;15:1453886. doi: 10.3389/fimmu.2024.1453886. eCollection 2024. Front Immunol. 2024. PMID: 39544945 Free PMC article. Review. - Immune dynamics shaping pre-metastatic and metastatic niches in liver metastases: from molecular mechanisms to therapeutic strategies.
Zhu C, Liao JY, Liu YY, Chen ZY, Chang RZ, Chen XP, Zhang BX, Liang JN. Zhu C, et al. Mol Cancer. 2024 Nov 14;23(1):254. doi: 10.1186/s12943-024-02171-z. Mol Cancer. 2024. PMID: 39543660 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources