The Role of TEG Analysis in Patients with COVID-19-Associated Coagulopathy: A Systematic Review - PubMed (original) (raw)
Review
The Role of TEG Analysis in Patients with COVID-19-Associated Coagulopathy: A Systematic Review
Jan Hartmann et al. Diagnostics (Basel). 2021.
Abstract
Coronavirus disease 2019 (COVID-19)-associated coagulopathy (CAC), characterized by hypercoagulability and an increased risk of thrombotic complications, is an important consideration in the management of patients with COVID-19. As COVID-19 is a new disease, no standard of care for the diagnosis or management of its associated coagulopathy is yet established. Whole blood viscoelastic tests, such as thromboelastography (TEG® hemostasis analyzer), analyze whole blood to provide a complete overview of the coagulation status. We conducted a systematic review of thromboelastography for management of patients with COVID-19, using MEDLINE (PubMed) and Cochrane databases. TEG® parameter measurements and clinical outcomes data were extracted for analysis. Our review found 15 publications, with overall results showing thromboelastography can identify and assess a hypercoagulable state in patients with COVID-19. Furthermore, utilization of thromboelastography in this patient population was shown to predict thrombotic complications. The benefits of thromboelastography presented here, in addition to advantages compared with laboratory coagulation tests, position thromboelastography as an important opportunity for optimizing diagnosis of CAC and improving patient management in COVID-19. Given that the benefits of thromboelastography have already been demonstrated in several other clinical applications, we anticipate that clinical data from future studies in patients with COVID-19 will further elucidate the optimal use of thromboelastography in this patient population.
Keywords: COVID-19; TEG; blood coagulation; coronavirus; fibrinolysis; thromboelastography; viscoelastic.
Conflict of interest statement
J.H., A.E., D.M. and J.D.D. were employees of Haemonetics at the time of the study. The roles of each author in the study design, data collection, analysis and interpretation, and the writing of the manuscript are given in the Author Contributions section above.
Figures
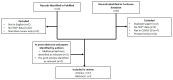
Figure 1
PRISMA diagram showing articles identified for inclusion in the review. COVID-19, coronavirus disease 2019; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TEG, thromboelastography.
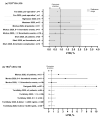
Figure 2
TEG MA values observed in patients with COVID-19. Grey bars show the normal TEG reference range [10]. CK, citrated kaolin assay; CFF, citrated functional fibrinogen assay; CKH, CK with heparinase assay; MA, maximum amplitude. * TEG measurements recorded before and after endovascular stent graft exclusion of the aortic thrombus and right lower limb embolectomy following diagnosis of acute ischemic limb. † Twice weekly TEG measurements recorded two weeks after the introduction of high-dose pharmacological thrombosis prophylaxis. ‡ Measurements were repeated on two consecutive days in six patients. Data points are mean with standard deviation error bars for normally distributed data, or median with interquartile range error bars for data that were not normally distributed. Dotted lines separate different studies with different patient populations. Data are not directly comparable between studies.
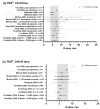
Figure 3
TEG LY30 values observed in patients with COVID-19. Grey bar shows the normal TEG reference range [10]. CK, citrated kaolin assay; CKH, CK with heparinase assay; LY30, clot lysis at 30 min after maximum clot strength. * TEG measurements recorded before and after endovascular stent graft exclusion of the aortic thrombus and right lower limb embolectomy following diagnosis of acute ischemic limb. † Measurements were repeated on two consecutive days in six patients. ‡ LY30 range reported: 0–54.3%. Data points are mean with standard deviation error bars for normally distributed data, or median with interquartile range error bars for data that were not normally distributed. Dotted lines separate different studies with different patient populations. Data are not directly comparable between studies.
Figure 4
TEG R-time values observed in patients with COVID-19. Grey bars show the normal TEG reference range [10]. CK, citrated kaolin assay; CKH, CK with heparinase assay; R-time, reaction time. * TEG measurements recorded before and after endovascular stent graft exclusion of the aortic thrombus and right lower limb embolectomy following diagnosis of acute ischemic limb. † Measurements were repeated on two consecutive days in six patients. Data points are mean with standard deviation error bars for normally distributed data, or median with interquartile range error bars for data that were not normally distributed. Dotted lines separate different studies with different patient populations. Data are not directly comparable between studies.
Similar articles
- Utility of Thromboelastography and velocity curve derivative in diagnosing COVID-19 associated coagulopathy.
Mohan G, Wilson W, Paonam B, Vaidya A, Ravindra P, Shastry S, Balakrishnan JM, Rao S, Chaudhuri S. Mohan G, et al. Int J Lab Hematol. 2022 Oct;44(5):823-830. doi: 10.1111/ijlh.13876. Epub 2022 May 24. Int J Lab Hematol. 2022. PMID: 35609623 Free PMC article. - Questions about COVID-19 associated coagulopathy: possible answers from the viscoelastic tests.
Pavoni V, Gianesello L, Pazzi M, Dattolo P, Prisco D. Pavoni V, et al. J Clin Monit Comput. 2022 Feb;36(1):55-69. doi: 10.1007/s10877-021-00744-7. Epub 2021 Jul 15. J Clin Monit Comput. 2022. PMID: 34264472 Free PMC article. Review. - Thromboelastography Profile of Patients with COVID-19 Admitted to Intensive Care Unit: A Single-center Retrospective Study from India.
Saseedharan S, Talla VB, Chiluka A. Saseedharan S, et al. Indian J Crit Care Med. 2020 Dec;24(12):1218-1222. doi: 10.5005/jp-journals-10071-23675. Indian J Crit Care Med. 2020. PMID: 33446976 Free PMC article. - Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis.
Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Whiting P, et al. Health Technol Assess. 2015 Jul;19(58):1-228, v-vi. doi: 10.3310/hta19580. Health Technol Assess. 2015. PMID: 26215747 Free PMC article. Review. - Thromboelastography determined dynamics of blood coagulation and its correlation with complications and outcomes in patients with coronavirus disease 2019.
Sehgal T, Aggarwal M, Baitha U, Gupta G, Prakash B, Gupta A, Kumar G, Biswas A, Khan M, Shalimar. Sehgal T, et al. Res Pract Thromb Haemost. 2022 Jan 15;6(1):e12645. doi: 10.1002/rth2.12645. eCollection 2022 Jan. Res Pract Thromb Haemost. 2022. PMID: 35071969 Free PMC article.
Cited by
- Modified Thromboelastography for Peri-interventional Assessment of Platelet Function in Cardiology Patients: A Narrative Review.
Hartmann J, Curzen N. Hartmann J, et al. Semin Thromb Hemost. 2023 Mar;49(2):192-200. doi: 10.1055/s-0042-1757545. Epub 2022 Oct 17. Semin Thromb Hemost. 2023. PMID: 36252602 Free PMC article. Review. - Rotational Thromboelastometry (ROTEM®) in Relation to Inflammatory Biomarkers and Clinical Outcome in COVID-19 Patients.
Rogalski P, Rogalska M, Martonik D, Rusak M, Pawlus J, Chociej-Stypulkowska J, Dabrowska M, Flisiak R. Rogalski P, et al. J Clin Med. 2023 Jun 8;12(12):3919. doi: 10.3390/jcm12123919. J Clin Med. 2023. PMID: 37373613 Free PMC article. - Clot Stiffness Measured By Seer Sonorheometry As a Marker Of Poor Prognosis In Hospitalized COVID-19 Patients.
López-Jaime FJ, Fernández-Bello I, Martín-Téllez S, Doblas-Márquez A, Tesfay Y, Márquez-Gómez I, Reguera-Iglesias JM, Muñoz-Pérez MI, Montaño A. López-Jaime FJ, et al. Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221112085. doi: 10.1177/10760296221112085. Clin Appl Thromb Hemost. 2022. PMID: 35903939 Free PMC article. - Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices.
Volod O, Bunch CM, Zackariya N, Moore EE, Moore HB, Kwaan HC, Neal MD, Al-Fadhl MD, Patel SS, Wiarda G, Al-Fadhl HD, McCoy ML, Thomas AV, Thomas SG, Gillespie L, Khan RZ, Zamlut M, Kamphues P, Fries D, Walsh MM. Volod O, et al. J Clin Med. 2022 Feb 7;11(3):860. doi: 10.3390/jcm11030860. J Clin Med. 2022. PMID: 35160311 Free PMC article. Review. - Thromboelastography: a review for radiologists and implications on periprocedural bleeding risk.
Willis J, Carroll C, Planz V, Galgano SJ. Willis J, et al. Abdom Radiol (NY). 2022 Aug;47(8):2697-2703. doi: 10.1007/s00261-022-03539-9. Epub 2022 May 14. Abdom Radiol (NY). 2022. PMID: 35567618 Free PMC article. Review.
References
- Rubulotta F., Soliman-Aboumarie H., Filbey K., Geldner G., Kuck K., Ganau M., Hemmerling T.M. Technologies to Optimize the Care of Severe COVID-19 Patients for Health Care Providers Challenged by Limited Resources. Anesth. Analg. 2020;131:351–364. doi: 10.1213/ANE.0000000000004985. - DOI - PMC - PubMed
- Shah A., Donovan K., McHugh A., Pandey M., Aaron L., Bradbury C.A., Stanworth S.J., Alikhan R., Von Kier S., Maher K., et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: A multicentre observational study. Crit. Care. 2020;24:561. doi: 10.1186/s13054-020-03260-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials