Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines - PubMed (original) (raw)
Review
Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines
Basmah N Aldosari et al. Pharmaceutics. 2021.
Abstract
There has been increased interest in the development of RNA-based vaccines for protection against various infectious diseases and also for cancer immunotherapies. Rapid and cost-effective manufacturing methods in addition to potent immune responses observed in preclinical and clinical studies have made mRNA-based vaccines promising alternatives to conventional vaccine technologies. However, efficient delivery of these vaccines requires that the mRNA be protected against extracellular degradation. Lipid nanoparticles (LNPs) have been extensively studied as non-viral vectors for the delivery of mRNA to target cells because of their relatively easy and scalable manufacturing processes. This review highlights key advances in the development of LNPs and reviews the application of mRNA-based vaccines formulated in LNPs for use against infectious diseases and cancer.
Keywords: adjuvant; cancer immunotherapy; cationic lipids; delivery system; lipid nanoparticles; mRNA; nanotechnology; nucleic acid; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
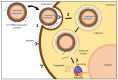
Figure 1
Intracellular barriers for in vitro transcribed (IVT) mRNA delivery: (1) interaction between the delivery system and the cell membrane, (2) endocytosis, and (3) endosomal escape and release of the mRNA to start the translation process (reproduced from Gomez-Aguado et al. [23]).
Figure 2
Key lipid nanocarriers of mRNA: (A) liposome, lipoplex, and lipid nanoparticle; (B) nanostructured lipid carrier; (C) cationic nanoemulsion (reproduced and modified from Granot et al. [53]).
Figure 3
Schematic representation of mRNA lipid nanoparticles (reproduced from Sempler et al. [61]). DOTMA: 1,2-di-O-octadecenyl-3-trimethylammonium propane; DOTAP: 1,2-dioleoyl-3-trimethylammonium-propane; MC3: D-Lin-MC3-DMA; DSPC: 1,2-distearoyl-sn-glycero-3-phosphocholine; DPPC: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPE-PEG: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol; DMPE-PEG: 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine polyethylene glycol; DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine.
Similar articles
- Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.
Qiu M, Li Y, Bloomer H, Xu Q. Qiu M, et al. Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review. - mRNA delivery technologies: Toward clinical translation.
Gómez-Aguado I, Rodríguez-Castejón J, Beraza-Millor M, Rodríguez-Gascón A, Del Pozo-Rodríguez A, Solinís MÁ. Gómez-Aguado I, et al. Int Rev Cell Mol Biol. 2022;372:207-293. doi: 10.1016/bs.ircmb.2022.04.010. Epub 2022 Jul 7. Int Rev Cell Mol Biol. 2022. PMID: 36064265 Review. - Chemistry of Lipid Nanoparticles for RNA Delivery.
Eygeris Y, Gupta M, Kim J, Sahay G. Eygeris Y, et al. Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review. - Lipid Microparticles Show Similar Efficacy With Lipid Nanoparticles in Delivering mRNA and Preventing Cancer.
Ji A, Xu M, Pan Y, Diao L, Ma L, Qian L, Cheng J, Liu M. Ji A, et al. Pharm Res. 2023 Jan;40(1):265-279. doi: 10.1007/s11095-022-03445-1. Epub 2022 Nov 30. Pharm Res. 2023. PMID: 36451070 Free PMC article. - mRNA vaccine delivery using lipid nanoparticles.
Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. Reichmuth AM, et al. Ther Deliv. 2016;7(5):319-34. doi: 10.4155/tde-2016-0006. Ther Deliv. 2016. PMID: 27075952 Free PMC article. Review.
Cited by
- Nanocarriers for delivery of siRNA as gene silencing mediator.
Morales-Becerril A, Aranda-Lara L, Isaac-Olivé K, Ocampo-García BE, Morales-Ávila E. Morales-Becerril A, et al. EXCLI J. 2022 Aug 1;21:1028-1052. doi: 10.17179/excli2022-4975. eCollection 2022. EXCLI J. 2022. PMID: 36110562 Free PMC article. Review. - Research advance in lipid nanoparticle-mRNA delivery system and its application in CAR-T cell therapy.
Ye B, Hu Y, Zhang M, Huang H. Ye B, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Apr 25;51(2):185-191. doi: 10.3724/zdxbyxb-2022-0047. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36161298 Free PMC article. Review. English. - RNA therapeutics in the clinic.
Curreri A, Sankholkar D, Mitragotri S, Zhao Z. Curreri A, et al. Bioeng Transl Med. 2022 Jul 6;8(1):e10374. doi: 10.1002/btm2.10374. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684099 Free PMC article. Review. - Smart pH-responsive nanomedicines for disease therapy.
Shinn J, Kwon N, Lee SA, Lee Y. Shinn J, et al. J Pharm Investig. 2022;52(4):427-441. doi: 10.1007/s40005-022-00573-z. Epub 2022 May 9. J Pharm Investig. 2022. PMID: 35573320 Free PMC article. Review. - Synthesis and Potential Applications of Lipid Nanoparticles in Medicine.
Musielak E, Feliczak-Guzik A, Nowak I. Musielak E, et al. Materials (Basel). 2022 Jan 17;15(2):682. doi: 10.3390/ma15020682. Materials (Basel). 2022. PMID: 35057398 Free PMC article. Review.
References
- Brito L.A., Kommareddy S., Maione D., Uematsu Y., Giovani C., Scorza F.B., Otten G.R., Yu D., Mandl C.W., Mason P.W. Advances in Genetics. Volume 89. Elsevier; Amsterdam, The Netherlands: 2015. Self-amplifying mRNA vaccines; pp. 179–233. - PubMed
- Pascolo S. Toll-Like Receptors (TLRs) and Innate Immunity. Springer; Berlin, Germany: 2008. Vaccination with messenger RNA (mRNA) pp. 221–235. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources