Alterations in extracellular matrix composition during aging and photoaging of the skin - PubMed (original) (raw)
Alterations in extracellular matrix composition during aging and photoaging of the skin
Maxwell C McCabe et al. Matrix Biol Plus. 2020.
Abstract
Human skin is composed of the cell-rich epidermis, the extracellular matrix (ECM) rich dermis, and the hypodermis. Within the dermis, a dense network of ECM proteins provides structural support to the skin and regulates a wide variety of signaling pathways which govern cell proliferation and other critical processes. Both intrinsic aging, which occurs steadily over time, and extrinsic aging (photoaging), which occurs as a result of external insults such as solar radiation, cause alterations to the dermal ECM. In this study, we utilized both quantitative and global proteomics, alongside single harmonic generation (SHG) and two-photon autofluorescence (TPAF) imaging, to assess changes in dermal composition during intrinsic and extrinsic aging. We find that both intrinsic and extrinsic aging result in significant decreases in ECM-supporting proteoglycans and structural ECM integrity, evidenced by decreasing collagen abundance and increasing fibril fragmentation. Intrinsic aging also produces changes distinct from those produced by photoaging, including reductions in elastic fiber and crosslinking enzyme abundance. In contrast, photoaging is primarily defined by increases in elastic fiber-associated protein and pro-inflammatory proteases. Changes associated with photoaging are evident even in young (mid 20s) sun-exposed forearm skin, indicating that proteomic evidence of photoaging is present decades prior to clinical signs of photoaging. GO term enrichment revealed that both intrinsic aging and photoaging share common features of chronic inflammation. The proteomic data has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD015982.
Keywords: AUC, area under the curve; Aging; CE, cornified envelope; CNBr, cyanogen bromide; Collagen; ECM, extracellular matrix; Extracellular matrix; GO, gene ontology; Photoaging; Proteomics; QconCATs, quantitative concatemers; SHG, single harmonic generation; Skin; TPAF, two-photon autofluorescence; UV, ultraviolet; iECM, insoluble ECM; sECM, soluble ECM.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Fig. 1
Experimental overview. Skin punch biopsies were collected from the hip, underarm, and forearm of young and aged subjects. Skin samples were subjected to compartment-resolved extraction, generating cellular (soluble), soluble ECM (sECM), and insoluble ECM (iECM) fractions. Each fraction was analyzed via mass spectrometry and 2129 proteins were identified across all samples at 1% FDR. Skin biopsies were additionally subjected to SHG and TPAF imaging to visualize collagen and elastin within the dermis.
Fig. 2
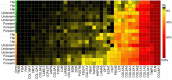
Solubility profiling of ECM components. Solubility of each protein is calculated as the percentage of total AUC ion intensity across all fractions which resulted from the iECM fraction. Rows represent hip, underarm, and forearm samples from young (green) and aged (orange) subjects.
Fig. 3
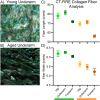
SHG/TFAP images for young (A) and aged underarm (B) showing collagen (white), elastin (green), and DAPI stain (blue). Collagen fiber length (C) and width (D) for young (green bar) and aged (orange bar) samples derived from SHG imaging using CT-FIRE image analysis. Error bars display 95% confidence interval for the sample group.
Fig. 4
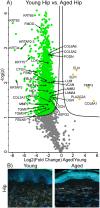
Differentially expressed proteins and ECM network alterations during intrinsic aging in human hip skin. Volcano plot visualization of significantly enriched proteins between young and aged hip skin (A). Proteins above significance cutoff colored green (young) or orange (aged). SHG/TFAP imaging of young and aged hip skin (B) showing collagen fibrils (white), elastin (green), and nuclei (blue).
Fig. 5
Absolute quantitation of key ECM proteins in young (green) and aged (orange) samples. Quantification was performed using SIL QconCAT peptide standards. Error bars represent standard deviation.
Fig. 6
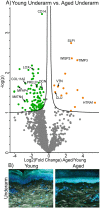
Differentially expressed proteins and ECM network alterations during intrinsic aging in human underarm skin. Volcano plot visualization of significantly enriched proteins between young and aged underarm skin (A). Proteins above significance cutoff colored green (young) or orange (aged). SHG/TFAP imaging of young and aged underarm skin (B) showing collagen fibrils (white), elastin (green), and nuclei (blue).
Fig. 7
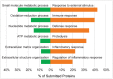
Selected gene ontology (GO) biological process terms enriched in young underarm (green) and aged underarm (orange). All proteins with p < 0.05 or FC > 3 between sample groups were selected for GO enrichment analysis. Displayed terms were selected due to high enrichment significance and relevance.
Fig. 8
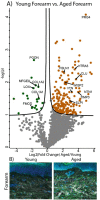
Differentially expressed proteins and ECM network alterations during intrinsic aging in human forearm skin. Volcano plot visualization of significantly enriched proteins between young and aged forearm skin (A). Proteins above significance cutoff colored green (young) or orange (aged). SHG/TFAP imaging of young and aged forearm skin (B) showing collagen fibrils (white), elastin (green), and nuclei (blue).
Fig. 9
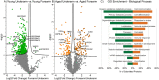
Differentially expressed proteins and network alterations during photoaging in human arm skin. Volcano plot visualization of significantly enriched proteins between young underarm and forearm skin (A) and aged underarm and forearm skin (B). Significantly enriched proteins are colored light green (underarm) or dark green (forearm) (A) and light orange (underarm) or dark orange (forearm) (B). Selected gene ontology (GO) biological process terms enriched in underarm (green) and forearm (orange) (C). Forearm-enriched and underarm-enriched proteins in both young and aged sample groups were pooled into two anatomical groups and all proteins with p < 0.05 or FC > 3 between groups were selected for GO enrichment analysis. Displayed terms were selected due to high enrichment significance and relevance.
Fig. 10
Summary of matrix alterations during intrinsic aging and photoaging. Representative SHG images of hip and forearm skin from young and aged subjects showing collagen fibrils (white), elastin (green), and nuclei (blue). Bars display change in abundance or solubility of ECM components during intrinsic aging and photoaging (darker, higher magnitude; lighter, lower magnitude).
Similar articles
- Physical properties of the photodamaged human skin dermis: Rougher collagen surface and stiffer/harder mechanical properties.
Shao Y, Qin Z, Alexander Wilks J, Balimunkwe RM, Fisher GJ, Voorhees JJ, Quan T. Shao Y, et al. Exp Dermatol. 2019 Aug;28(8):914-921. doi: 10.1111/exd.13728. Epub 2018 Jul 29. Exp Dermatol. 2019. PMID: 29957839 Free PMC article. - Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations.
Hwang KA, Yi BR, Choi KC. Hwang KA, et al. Lab Anim Res. 2011 Mar;27(1):1-8. doi: 10.5625/lar.2011.27.1.1. Epub 2011 Mar 25. Lab Anim Res. 2011. PMID: 21826153 Free PMC article. - Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation.
Parkinson LG, Toro A, Zhao H, Brown K, Tebbutt SJ, Granville DJ. Parkinson LG, et al. Aging Cell. 2015 Feb;14(1):67-77. doi: 10.1111/acel.12298. Epub 2014 Dec 11. Aging Cell. 2015. PMID: 25495009 Free PMC article. - The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure.
Uitto J. Uitto J. J Drugs Dermatol. 2008 Feb;7(2 Suppl):s12-6. J Drugs Dermatol. 2008. PMID: 18404866 Review.
Cited by
- Biochemical, structural and physical changes in aging human skin, and their relationship.
Park S. Park S. Biogerontology. 2022 Jun;23(3):275-288. doi: 10.1007/s10522-022-09959-w. Epub 2022 Mar 15. Biogerontology. 2022. PMID: 35292918 Free PMC article. Review. - SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway.
Ham SM, Song MJ, Yoon HS, Lee DH, Chung JH, Lee ST. Ham SM, et al. Int J Mol Sci. 2023 Jul 29;24(15):12179. doi: 10.3390/ijms241512179. Int J Mol Sci. 2023. PMID: 37569556 Free PMC article. - Infiltration of subcutaneous adipose layer into the dermal layer with aging.
Ezure T, Amano S, Matsuzaki K. Ezure T, et al. Skin Res Technol. 2022 Mar;28(2):311-316. doi: 10.1111/srt.13133. Epub 2022 Jan 12. Skin Res Technol. 2022. PMID: 35020969 Free PMC article. - Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts.
Kim KS, Son SR, Choi YJ, Kim Y, Ahn SY, Jang DS, Lee S. Kim KS, et al. Plants (Basel). 2024 May 2;13(9):1266. doi: 10.3390/plants13091266. Plants (Basel). 2024. PMID: 38732481 Free PMC article. - Different epigenetic signatures of newborn telomere length and telomere attrition rate in early life.
Wang C, Nawrot TS, Van Der Stukken C, Tylus D, Sleurs H, Peusens M, Alfano R, Langie SAS, Plusquin M, Martens DS. Wang C, et al. Aging (Albany NY). 2021 Jun 4;13(11):14630-14650. doi: 10.18632/aging.203117. Epub 2021 Jun 4. Aging (Albany NY). 2021. PMID: 34086604 Free PMC article.
References
- Wlaschek M., Tantcheva-Poor I., Naderi L., Ma W., Schneider L.A., Razi-Wolf Z., Schuller J., Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001;63:41–51. www.elsevier.com - PubMed
- Fisher G.J., Kang S., Varani J., Bata-csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Arch Dermatology. 2002;138:1462–1470. - PubMed
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J. Drugs Dermatology. 2008;7:s12–s16. http://www.ncbi.nlm.nih.gov/pubmed/18404866 - PubMed
LinkOut - more resources
Full Text Sources