Long non-coding RNAs: novel players in the pathogenesis of polycystic ovary syndrome - PubMed (original) (raw)
Review
Long non-coding RNAs: novel players in the pathogenesis of polycystic ovary syndrome
Mixue Tu et al. Ann Transl Med. 2021 Jan.
Abstract
Long non-coding RNAs (lncRNAs) are a class of transcripts (>200 nucleotides) lacking protein-coding capacity. Based on the complex three-dimensional structure, lncRNAs are involved in many biological processes and can regulate the expression of target genes at chromatin modification, transcriptional and post-transcriptional levels. LncRNAs have been studied in multiple diseases but little is known about their role(s) in polycystic ovary syndrome (PCOS), the most common endocrinological disorder in reproductive-aged women around the world. In this review, we characterized and explored the potential mechanisms of lncRNAs in the pathogenesis of PCOS. We found that lncRNAs play a molecular role in PCOS mainly by functioning as the competitive endogenous RNA (ceRNA) and are significantly correlated with some clinical phenotypes. We summarized in detail regarding aberrant lncRNAs in different specimens of women with PCOS [i.e., granulosa cells (GCs), cumulus cells (CCs), follicular fluid (FF), peripheral blood] and various PCOS rodent models [i.e., dehydroepiandrosterone (DHEA) and letrozole induced models]. In clinical practice, detection of lncRNAs in serum might enable early diagnosis. Furthermore, new lncRNA-based classifications might be emerging as potent predictors of a particular phenotype in PCOS. Overall, we proposed new insights for the application of precision medicine approaches to the management of PCOS.
Keywords: Long non-coding RNAs; cumulus cells (CCs); granulosa cells (GCs); oocyte; polycystic ovary syndrome (PCOS).
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/atm-20-5044). Dr. DZ, Dr. LM, Dr. YW, and MT report grants from National Key Research & Developmental Program of China, grants from National Natural Science Foundation of China, during the conduct of the study.
Figures
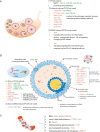
Figure 1
Identified lncRNA in PCOS. (A) LncRNA profile are depicted in ovarian issue from PCOS patients and rodent model. The function of most listed lncRNAs has not been investigated; (B) LncRNA signatures in follicles are key for uncovering the pathophysiologic mechanism in PCOS. The studies in granulosa cells are the most, and those lncRNA work maily through ceRNA network and some other identified singaling pathways (i.e., p53 pathway, NF-κB pathway). Little is know about the lncRNA function in follicular fluid. Therefore, we hypothesis that lncRNAs are transported from GCs to oocyte via extracellular vesicles, regulating the gene expression in oocyte during the follicle development. Several lncRNA are reported to be involved in the development of human follicle and regulate the transcription and RNA processing in paraspeckle, but the blanks about the lncRNAs signature in oocyte of PCOS urgently need to be filled in to better understand the molecular mechanism of ovarian follicular arrest; (C) LncRNA detected in peripherial blood are associated with endocrine changes (i.e., lipid metabolism, androgen metabolism, glucose or insulin metabolism) and even connected with the pregnant outcome, providing new insights for our further understand of lncRNAs in PCOS and expand the novel diagnostic biomarkers and therapeutic targets.
Similar articles
- Aberrant expression of long noncoding RNAs in cumulus cells isolated from PCOS patients.
Huang X, Hao C, Bao H, Wang M, Dai H. Huang X, et al. J Assist Reprod Genet. 2016 Jan;33(1):111-21. doi: 10.1007/s10815-015-0630-z. Epub 2015 Dec 9. J Assist Reprod Genet. 2016. PMID: 26650608 Free PMC article. - Long Noncoding RNAs: Potential Regulators Involved in the Pathogenesis of Polycystic Ovary Syndrome.
Liu YD, Li Y, Feng SX, Ye DS, Chen X, Zhou XY, Chen SL. Liu YD, et al. Endocrinology. 2017 Nov 1;158(11):3890-3899. doi: 10.1210/en.2017-00605. Endocrinology. 2017. PMID: 28938484 - Long non-coding RNAs in ovarian granulosa cells.
Tu J, Chen Y, Li Z, Yang H, Chen H, Yu Z. Tu J, et al. J Ovarian Res. 2020 Jun 5;13(1):63. doi: 10.1186/s13048-020-00663-2. J Ovarian Res. 2020. PMID: 32503679 Free PMC article. Review. - MicroRNAs related to androgen metabolism and polycystic ovary syndrome.
Sørensen AE, Udesen PB, Wissing ML, Englund ALM, Dalgaard LT. Sørensen AE, et al. Chem Biol Interact. 2016 Nov 25;259(Pt A):8-16. doi: 10.1016/j.cbi.2016.06.008. Epub 2016 Jun 5. Chem Biol Interact. 2016. PMID: 27270454 Review.
Cited by
- Combined Transcriptomic and Metabolomic Analysis of Women with Polycystic Ovary Syndrome.
Tian-Min Y, Suxia L, Shufang D, Dandan C, Long-Dan L, Shu Biu YW. Tian-Min Y, et al. Dis Markers. 2022 Aug 28;2022:4000424. doi: 10.1155/2022/4000424. eCollection 2022. Dis Markers. 2022. PMID: 36072900 Free PMC article. - Identification of Three Potential circRNA Biomarkers of Polycystic Ovary Syndrome by Bioinformatics Analysis and Validation.
Huang P, Du S, Lin Y, Huang Z, Li H, Chen G, Chen S, Chen Q, Da L, Shi H, Wei W, Yang L, Sun Y, Zheng B. Huang P, et al. Int J Gen Med. 2021 Sep 22;14:5959-5968. doi: 10.2147/IJGM.S324126. eCollection 2021. Int J Gen Med. 2021. PMID: 34588800 Free PMC article. - Biomarkers in Polycystic Ovary Syndrome.
Huffman AM, Rezq S, Basnet J, Romero DG. Huffman AM, et al. Curr Opin Physiol. 2023 Dec;36:100717. doi: 10.1016/j.cophys.2023.100717. Epub 2023 Sep 18. Curr Opin Physiol. 2023. PMID: 37842179 Free PMC article. - Follicular fluid-derived exosomal LncRNA LIPE-AS1 modulates steroid metabolism and survival of granulosa cells leading to oocyte maturation arrest in polycystic ovary syndrome.
Yu L, Wang C, Liu M, Xia L, Liu T, Che Q, Cai W, Dong X, Pan B, Wang B, Liu S, Guo W. Yu L, et al. J Assist Reprod Genet. 2024 May;41(5):1387-1401. doi: 10.1007/s10815-024-03092-y. Epub 2024 Apr 24. J Assist Reprod Genet. 2024. PMID: 38656738 - Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome.
Cao J, Huo P, Cui K, Wei H, Cao J, Wang J, Liu Q, Lei X, Zhang S. Cao J, et al. Cell Commun Signal. 2022 May 9;20(1):61. doi: 10.1186/s12964-022-00876-6. Cell Commun Signal. 2022. PMID: 35534864 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources