Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo - PubMed (original) (raw)
. 2021 May 7;42(18):1760-1769.
doi: 10.1093/eurheartj/ehab027.
Charlotte Rasser 2, Jules Mesnier 2, Pascale Chevallier 1, Romain Gallet 3, Christine Choqueux 2, Guillaume Even 2, Neila Sayah 2, Frédéric Chaubet 2, Antonino Nicoletti 2, Bijan Ghaleh 3, Laurent J Feldman 2 4, Diego Mantovani 1, Giuseppina Caligiuri 2 4
Affiliations
- PMID: 33580685
- PMCID: PMC8106951
- DOI: 10.1093/eurheartj/ehab027
Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo
Sergio Diaz-Rodriguez et al. Eur Heart J. 2021.
Abstract
Aims: The rapid endothelialization of bare metal stents (BMS) is counterbalanced by inflammation-induced neointimal growth. Drug-eluting stents (DES) prevent leukocyte activation but impair endothelialization, delaying effective device integration into arterial walls. Previously, we have shown that engaging the vascular CD31 co-receptor is crucial for endothelial and leukocyte homeostasis and arterial healing. Furthermore, we have shown that a soluble synthetic peptide (known as P8RI) acts like a CD31 agonist. The aim of this study was to evaluate the effect of CD31-mimetic metal stent coating on the in vitro adherence of endothelial cells (ECs) and blood elements and the in vivo strut coverage and neointimal growth.
Methods and results: We produced Cobalt Chromium discs and stents coated with a CD31-mimetic peptide through two procedures, plasma amination or dip-coating, both yielding comparable results. We found that CD31-mimetic discs significantly reduced the extent of primary human coronary artery EC and blood platelet/leukocyte activation in vitro. In vivo, CD31-mimetic stent properties were compared with those of DES and BMS by coronarography and microscopy at 7 and 28 days post-implantation in pig coronary arteries (n = 9 stents/group/timepoint). Seven days post-implantation, only CD31-mimetic struts were fully endothelialized with no activated platelets/leukocytes. At day 28, neointima development over CD31-mimetic stents was significantly reduced compared to BMS, appearing as a normal arterial media with the absence of thrombosis contrary to DES.
Conclusion: CD31-mimetic coating favours vascular homeostasis and arterial wall healing, preventing in-stent stenosis and thrombosis. Hence, such coatings seem to improve the metal stent biocompatibility.
Keywords: Biocompatibility; Biomimetic device; CD31; Coronary; Endothelium; Stent.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
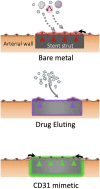
CD31 mimetic coating favours the growth of a physiologic endothelial wall on stent struts: bare metal, drug-eluting and CD31-mimetic.
Figure 1
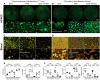
In vitro analysis of human coronary artery endothelial cells and blood elements adhering to CD31-mimetic Cobalt Chromium discs. (A_–_D) Representative digital fluorescent images of human coronary artery endothelial cells and human blood elements interacting with Cobalt Chromium discs obtained by either plasma amination (A, C) or dip-coating (B, D) procedures. Controls for plasma amination included discs subjected to electropolishing alone (bare) or completed with poly(ethylene glycol) bis(carboxymethyl) ether linker covalent binding. Control discs for dip-coating were subjected to ultrasound cleaning in acidic water (bare) or completed with the self-assembly of a thin polydopamine film. (E) Human coronary artery endothelial cells adhering on the experimental discs were quantified in terms of number/surface (count of nuclei, i.e. Hoechst staining, expressed as N/µm2) and of integrated density of CD31 at the cell–cell border (green, IntDens, arbitrary units). Both parameters were significantly greater in coated as compared to bare metal discs; the presence of the CD31 peptide tended to yield higher values compared to poly(ethylene glycol) bis(carboxymethyl) ether or polydopamine coatings, although the difference did not reach statistical significance. (F) Blood leukocytes and platelets were quantified individually (N/µm2) by analysing the fluorescent particles in the blue (nuclei = leukocytes, Hoechst+ particles) and orange (platelets = CD41+ particles) channels, respectively, and as aggregates (% of total elements) by counting the number of blue elements overlapping orange elements. von Willebrand factor+ immune staining within platelets (red in the left panel and green in the right panel) was also analysed. Of note, the number of von Willebrand factor+ elements yielded identical data as for the CD41+ element analysis (data not shown). Polydopamine and poly(ethylene glycol) bis(carboxymethyl) ether coatings were able to reduce blood leukocyte adherence and platelet-leukocyte aggregates (especially polydopamine) but, importantly, both failed to reduce platelet adherence and the latter was reduced only in the presence of the CD31 peptide. The indicated scale bars apply to all the images of the same row.
Figure 2
In vitro analysis of soluble markers released by human coronary artery endothelial cells and blood elements in contact with CD31-mimetic Cobalt Chromium discs. (A and B) Quantitative analysis of coagulation and inflammation soluble factors released in the supernatant of human coronary artery endothelial cells cultured during 48 h (A) or released in the blood-derived plasma after 1 h of incubation under continuous rotation (B), in the presence of CD31-mimetic Cobalt Chromium-coated discs or their controls. Individual points are median values from eight replicates of four different donors. The release of plasminogen activator-1, interleukin-6, interleukin-8, E-selectin in the supernatant of human coronary artery endothelial cells and the secretion of tissue factor, plasminogen activator-1, tissue factor pathway inhibitor, CD40L, macrophage inflammatory protein-1 alpha in the plasma derived from the blood incubated with the experimental discs was significantly reduced by the presence of the CD31 peptide, regardless of the type of linker arm used [i.e. poly(ethylene glycol) bis(carboxymethyl) ether or polydopamine]. Furthermore, human coronary artery endothelial cell release of soluble tissue factor pathway inhibitor was significantly increased in the presence of the CD31-mimetic peptide compared to bare and poly(ethylene glycol) bis(carboxymethyl) ether controls, reflecting a CD31-dependent physiologic anticoagulant function of the endothelium. ICAM-1, intercellular adhesion molecule-1; PDGF-BB, platelet-derived growth factor-BB; VCAM-1, vascular cell adhesion molecule 1. Circles: plasma amination; squares: dip-coating.
Figure 3
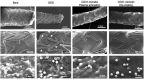
In vivo stent endothelialization at day 7 post-implantation. Representative examples of scanning electron microscopy imaging of the luminal surface of pig coronary arteries implanted with either bare metal or drug-eluting stent vs. CD31-mimetic plasma aminated or dip-coated stents (n = 9 stents/group), taken at low (top), medium (middle) or high (bottom) magnification. Arrowheads point at the absence of endothelialization over the drug-eluting stent struts. Note the presence of activated leukocytes (numerous pseudopods at their surface) tangled in a dense fibrin mesh over the endothelialized struts of bare metal stents. Non-activated (round-shaped) leukocytes can be identified over drug-eluting stent struts but there endothelial cells appeared detached one from another and covered with platelets and fibrin. Instead, the absence of leukocyte activation (round shape) was combined with the presence of a smooth and continuous endothelium, free from platelets and fibrin, over CD31-mimetic stent struts.
Figure 4
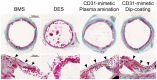
Morphometric analysis at day 7 post-stent implantation. Representative images of porcine coronary artery cross-sections observed at day 7 after stent implantation with either bare metal stent, drug-eluting stent, or CD31-mimetic stents (plasma aminated or dip-coated) and stained with Masson’s trichrome. Arrowheads indicate the presence of a continuous endothelial layer over the stent struts in the bare metal stent and CD31-mimetic groups, but not over the drug-eluting stent struts. The latter were also the only ones showing the presence of a fibrin layer (asterisks) and signs of necrosis (arrows) within the tissue matter covering the stent struts.
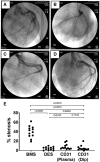
Figure 5
Evaluation of in-stent stenosis by coronary angiography at day 28. Representative angiography images of the left coronary arteries at 28 days after implantation of either bare metal stent (A), drug-eluting stent (B), plasma aminated (C), or dip-coated (D) CD31-mimetic stents. White arrows: proximal edge of the implanted stent. Yellow outlines: lumen shape within the stent. (E) Quantitative analysis of coronary stenosis (% lumen size reduction). The extent of stenosis was comparable in drug-eluting stent and CD31-mimetic stent groups and significantly lower compared to the bare metal stent group, regardless of the coronary artery type.
Figure 6
Morphometric analysis of the neointima at day 28. Composition of in-stent neointima at 28 days after stent implantation. (A) Masson’s trichrome staining. Black = nuclei, deep green = extracellular matrix, purple = cell cytoplasm, red = erythrocytes; light green/grey = ancient fibrin/platelets. Note the thick neointima and presence of leukocytes (black round nuclei) with the bare metal stent, the presence of fibrin, platelets, and erythrocytes with the drug-eluting stent and the organized media-like structure of the thin neointima with the CD31-mimetic stents. The black squares in low magnification images indicate the localization of the magnified insets. (B) Fluorescence microscopy analysis of Sirius Red-stained resin cross-sections of stented arteries. Red = collagen and elastin; green = stromal cell cytoplasm. White dotted line = internal elastic lamina. Note the consistent presence of stromal cells (green) in the media of arteries implanted with CD31-mimetic stents as compared to their paucity when implanted with drug-eluting stent and virtual absence with bare metal stent. Several stromal cells were also visible with bare metal stent but localized in the neointima (tissue growing inward of the stent struts and internal elastic lamina). (C) Quantitative analysis of neointima surface area (µm2). Individual points are median values from three sections (proximal, middle and distal cross-section) of each sample.
Comment in
- Refining drug-eluting stent technologies: from engineering to basic science.
Lansky A, Chun H, Pietras C, Hussain Y. Lansky A, et al. Eur Heart J. 2021 May 7;42(18):1770-1772. doi: 10.1093/eurheartj/ehab091. Eur Heart J. 2021. PMID: 33624813 No abstract available.
Similar articles
- Comparative preclinical evaluation of a polymer-free sirolimus-eluting stent in porcine coronary arteries.
Sperling C, Waliszewski MW, Kherad B, Krackhardt F. Sperling C, et al. Ther Adv Cardiovasc Dis. 2019 Jan-Dec;13:1753944719826335. doi: 10.1177/1753944719826335. Ther Adv Cardiovasc Dis. 2019. PMID: 30803407 Free PMC article. - CD31 Mimetic Coating Enhances Flow Diverting Stent Integration into the Arterial Wall Promoting Aneurysm Healing.
Cortese J, Rasser C, Even G, Bardet SM, Choqueux C, Mesnier J, Perrin ML, Janot K, Caroff J, Nicoletti A, Michel JB, Spelle L, Caligiuri G, Rouchaud A. Cortese J, et al. Stroke. 2021 Jan;52(2):677-686. doi: 10.1161/STROKEAHA.120.030624. Epub 2021 Jan 8. Stroke. 2021. PMID: 33412905 - The Genous™ endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries.
van Beusekom HM, Ertaş G, Sorop O, Serruys PW, van der Giessen WJ. van Beusekom HM, et al. Catheter Cardiovasc Interv. 2012 Feb 1;79(2):231-42. doi: 10.1002/ccd.22928. Epub 2011 Aug 10. Catheter Cardiovasc Interv. 2012. PMID: 21834062 - The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents.
Nakazawa G, Finn AV, John MC, Kolodgie FD, Virmani R. Nakazawa G, et al. Am J Cardiol. 2007 Oct 22;100(8B):36M-44M. doi: 10.1016/j.amjcard.2007.08.020. Am J Cardiol. 2007. PMID: 17950831 Review. - Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy.
Curcio A, Torella D, Indolfi C. Curcio A, et al. Circ J. 2011;75(6):1287-96. doi: 10.1253/circj.cj-11-0366. Epub 2011 Apr 29. Circ J. 2011. PMID: 21532177 Review.
Cited by
- The path to a hemocompatible cardiovascular implant: Advances and challenges of current endothelialization strategies.
Exarchos V, Zacharova E, Neuber S, Giampietro C, Motta SE, Hinkov H, Emmert MY, Nazari-Shafti TZ. Exarchos V, et al. Front Cardiovasc Med. 2022 Sep 14;9:971028. doi: 10.3389/fcvm.2022.971028. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36186971 Free PMC article. Review. - A TEMPOL and rapamycin loaded nanofiber-covered stent favors endothelialization and mitigates neointimal hyperplasia and local inflammation.
Wang R, Lu J, Yin J, Chen H, Liu H, Xu F, Zang T, Xu R, Li C, Wu Y, Wu Q, Fei X, Zhu M, Shen L, Ge J. Wang R, et al. Bioact Mater. 2022 May 11;19:666-677. doi: 10.1016/j.bioactmat.2022.04.033. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35600979 Free PMC article. - Comparison of Robot-Assisted Multivessel Minimally Invasive Direct Coronary Artery Bypass and Hybrid Revascularization.
Zhou Z, Dilip KA, Gleboff A, Nazem A, Green GR, Cherney A, Lutz CJ. Zhou Z, et al. Ann Thorac Surg Short Rep. 2023 Dec 19;2(2):226-230. doi: 10.1016/j.atssr.2023.11.029. eCollection 2024 Jun. Ann Thorac Surg Short Rep. 2023. PMID: 39790165 Free PMC article.
References
- Kornowski R, Hong MK, Virmani R, Jones R, Vodovotz Y, Leon MB. Granulomatous ‘foreign body reactions’ contribute to exaggerated in-stent restenosis. Coron Artery Dis 1999;10:9–14. - PubMed
- Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. - PubMed
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–1780. - PubMed
- Solinas E, Dangas G, Kirtane AJ, Lansky AJ, Franklin-Bond T, Boland P, Syros G, Kim YH, Gupta A, Mintz G, Fahy M, Collins M, Kodali S, Stone GW, Moses JW, Leon MB, Mehran R. Angiographic patterns of drug-eluting stent restenosis and one-year outcomes after treatment with repeated percutaneous coronary intervention. Am J Cardiol 2008;102:311–315. - PubMed
- Byrne RA, Iijima R, Mehilli J, Pinieck S, Bruskina O, Schomig A, Kastrati A. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv 2009;2:291–299. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources