Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers - PubMed (original) (raw)
Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers
Rujiu Hu et al. J Anim Sci Biotechnol. 2021.
Abstract
Background: Lactobacillus reuteri strains are widely used as probiotics to prevent and treat inflammatory bowel disease by modulating the host's immune system. However, the underlying mechanisms by which they communicate with the host have not been clearly understood. Bacterial extracellular vesicles (EVs) have been considered as important mediators of host-pathogen interactions, but their potential role in commensals-host crosstalk has not been widely studied. Here, we investigated the regulatory actions of EVs produced by L. reuteri BBC3, a gut-associated commensal bacterium of Black-Bone chicken, in the development of lipopolysaccharide (LPS)-induced intestinal inflammation in a chicken model using both in vivo and in vitro experiments.
Results: L. reuteri BBC3 produced nano-scale membrane vesicles with the size range of 60-250 nm. Biochemical and proteomic analyses showed that L. reuteri BBC3-derived EVs (LrEVs) carried DNA, RNA and several bioactive proteins previously described as mediators of other probiotics' beneficial effects such as glucosyltransferase, serine protease and elongation factor Tu. In vivo broiler experiments showed that administration of LrEVs exerted similar effects as L. reuteri BBC3 in attenuating LPS-induced inflammation by improving growth performance, reducing mortality and decreasing intestinal injury. LrEVs suppressed the LPS-induced expression of pro-inflammatory genes (TNF-α, IL-1β, IL-6, IL-17 and IL-8), and improved the expression of anti-inflammatory genes (IL-10 and TGF-β) in the jejunum. LrEVs could be internalized by chicken macrophages. In vitro pretreatment with LrEVs reduced the gene expression of TNF-α, IL-1β and IL-6 by suppressing the NF-κB activity, and enhanced the gene expression of IL-10 and TGF-β in LPS-activated chicken macrophages. Additionally, LrEVs could inhibit Th1- and Th17-mediated inflammatory responses and enhance the immunoregulatory cells-mediated immunosuppression in splenic lymphocytes of LPS-challenged chickens through the activation of macrophages. Finally, we revealed that the reduced content of both vesicular proteins and nucleic acids attenuated the suppression of LrEVs on LPS-induced inflammatory responses in ex vivo experiments, suggesting that they are essential for the LrEVs-mediated immunoregulation.
Conclusions: We revealed that LrEVs participated in maintaining intestinal immune homeostasis against LPS-induced inflammatory responses in a chicken model. Our findings provide mechanistic insight into how commensal and probiotic Lactobacillus species modulate the host's immune system in pathogens-induced inflammation.
Keywords: Chickens; Extracellular vesicles; Immune responses; Inflammation; Lactobacillus; Microbiota-host communication; Probiotics.
Conflict of interest statement
We declare that we have no competing interests.
Figures
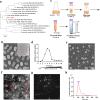
Fig. 1
Preparation and characterization of L. reuteri BBC3-derived EVs (LrEVs). a Phylogenetic diagram of L. reuteri BBC3 based on 16S rRNA sequences. The 15 most homologous sequences in the GenBank database were selected for the construction of a phylogenetic tree. b Representative image of the scanning electron microscope for L. reuteri BBC3 cells showing membrane vesicles on the bacterial cell surface. c Isolation and purification procedures of bacterial EVs. d LrEVs were purified by the discontinuous density ultracentrifugation. Nanoparticle tracking analysis (NTA) was performed to detect the particle numbers of each gradient fraction. Representative images of the scanning electron microscope (e) and transmission electron microscopy (f) for LrEVs. g Representative image from the recorded movies using a SCMOS camera of Malven NTA 3.0 when LrEVs were characterized by NTA. h Concentration and size distribution of the purified LrEVs determined by NTA
Fig. 2
Biochemical and proteomic analyses of LrEVs. a Quantifications of protein, DNA and RNA in the LrEVs. b Venn diagram showed that 92 overlapping proteins were identified in triplicate samples. c Subcellular localization of the identified proteins present in the LrEVs. It was found that 56.5% was cytoplasmic and 43.5% belonged to the membrane and secreted proteins. d Biological function classification of the identified proteins. It was found that the majority was either metabolic process (39.1%) or proteases and stress (19.6%). e A selected list of the identified proteins which may function in immune regulation, including protein accession, protein description and possible mechanisms
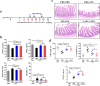
Fig. 3
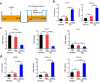
L. reuteri BBC3 and LrEVs attenuate lipopolysaccharide (LPS)-induced intestinal injury and inflammation in broiler chickens. a Experimental schedule for LPS challenge and administration of L. reuteri BBC3 and LrEVs. LPS (500 μg/bird) from E. coli O111: B4 was intraperitoneally injected 3 times; L. reuteri BBC3 (5 × 109 CFU/bird) and purified LrEVs (200 μg/bird) in 200 μL protectant (5% skim milk) were given 7 times by gavage. b Growth performance of each treatment from 7 to 21 days of age, including average daily weight gain (ADWG), average daily feed intake (ADFI), feed gain ratio (F/G) and mortality. c Representative image of each treatment group from hematoxylin and eosin-stained jejunum slides. d Intestinal morphology analysis based on measurements of villus height, crypt depth and the ratio of villus height to crypt depth (VH/CD) in jejunum tissues. Values are expressed as means ± SEM (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant
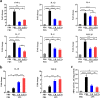
Fig. 4
LrEVs modulate the gene expression of pro- and anti-inflammatory mediators in jejunum tissues. a LrEVs suppressed the LPS-induced gene expression of pro-inflammatory mediators, including pro-inflammatory cytokine genes TNF-α, IL-1β, IL-6 and IL-17, and chemokine genes IL-8 and MIP-1β. b LrEVs enhanced the expression of anti-inflammatory cytokine genes IL-10 and TGF-β under the LPS-challenged condition. c LrEVs inhibited the LPS-induced activation of myeloperoxidase (MPO). Values are expressed as means ± SEM (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant
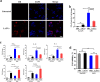
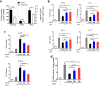
Fig. 5
LrEVs suppress LPS-induced inflammatory responses in chicken HD11 macrophages (Mφ). a Confocal microscopy showed that LrEVs were internalized by HD11 cells. The HD11 cells were co-incubated with medium (row 1) and DiI-labeled LrEVs (row 2) for 6 h at 37 °C. LrEVs were labeled with DiI (red signal), and the cell nucleus was stained with DAPI (blue signal). b NF-κB p65 transcription factor activity in the cell nucleus from HD11 cells treated with the indicated conditions. HD11 cells (5 × 105 cells/mL) were pretreated with PBS or LrEVs (10 μg/mL) for 12 h and stimulated with PBS or LPS (1 μg/mL) for 12 h. c The expression of pro-inflammatory cytokine genes TNF-α, IL-1β, IL-6 and anti-inflammatory cytokine genes IL-10 and TGF-β in HD11 cells treated with the indicated conditions. d Viability of HD11 cells determined by Trypan Blue dye exclusion assay. Data are representative of three independent experiments and expressed as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant
Fig. 6
Treatment of macrophages with LrEVs induces anti-inflammatory responses in splenic lymphocytes. a Experimental schedule of Mφ-splenic lymphocytes coculture in vitro using a Transwell system. HD11 cells (5 × 105 cells/mL) were pretreated with PBS or LrEVs (10 μg/mL) for 12 h, washed and added to the basolateral compartment of a 6-well plate. Splenic lymphocytes (5 × 105 cells/mL) from LPS-challenged chickens were seeded on the apical compartment of 6-well Hanging Inserts and incubated with or without PBS- or LrEVs-pretreated HD11 cells for 12 h. b LrEVs increased the expression of anti-inflammatory cytokine genes IL-10 and TGF-β in splenic lymphocytes of LPS-challenged chickens during in vitro coculture with HD11 cells. c LrEVs suppressed the expression of pro-inflammatory cytokine genes IFN-γ and IL-17 in splenic lymphocytes of LPS-challenged chickens during in vitro coculture with HD11 cells. d Treatment of HD11 cells with LrEVs improved the gene expression of CD25, T-lymphocyte antigen 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3). Data are representative of three independent experiments and expressed as means ± SEM. ***P < 0.001; NS, not significant
Fig. 7
The reduced content of vesicular proteins and nucleic acids decreased the anti-inflammatory effects of LrEVs. a The content of proteins, DNA or RNA in the LrEVs treated with proteinase K, DNase I or RNase I (see the method). b The expression of pro-inflammatory cytokine genes TNF-α, IL-6, IFN-γ and IL-17 in ex vivo jejunum explants. The explants were pretreated with native LrEVs (10 μg/mL), DNase I and RNase I-treated LrEVs (DR-LrEVs; 10 μg/mL) or proteinase K-agarose-treated LrEVs (PK-LrEVs; 10 μg/mL before proteinase K-agarose treatment) for 6 h and then stimulated with LPS (1 μg/mL) for 6 h. The gene expression of IL-10 and TGF-β (c) and MPO activity (d) in ex vivo jejunum explants. Data are representative of three independent experiments and expressed as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant
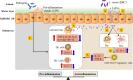
Fig. 8
Proposed mechanism of LrEVs-mediated bacteria-host crosstalk to drive the intestinal immune homeostasis against pathogens-induced inflammation in the chicken model. In inflammatory bowel disease, pathogens remarkably proliferate in the gut lumen and produce pro-inflammatory signals, such as lipopolysaccharide (LPS), which activate inflammatory cells, including macrophages (Mφ), Th1 and Th17 cells, to produce pro-inflammatory responses (1) [57]. L. reuteri BBC3 releases nanosized and highly biocompatible EVs that can drive the long-distance transport of interior molecules throughout the intracellular compartments in a concentrated, protected and targeted manner (2) [72]. These vesicles can suppress the pro-inflammatory mediators produced by inflammatory cells (activated Mφ) (3), and activate innate immune cells, including naïve Mφ (4) and possibly dendritic cells (DCs) (5), to produce immunoregulatory cytokines (possibly including IL-10) (6) that induce the development of immunoregulatory CD4+CD25+ cells. These resulting CD4+CD25+ cells can produce anti-inflammatory cytokines IL-10 and TGF-β that inhibit the production of pro-inflammatory cytokines (7) [62]. Further studies are required to investigate the potential interactions between LrEVs and other intestinal immune cells, especially DCs (5) and epithelial cells (8)
Similar articles
- Oral probiotic extracellular vesicle therapy mitigates Influenza A Virus infection via blunting IL-17 signaling.
Zhou H, Huang W, Li J, Chen P, Shen L, Huang W, Mai K, Zou H, Shi X, Weng Y, Liu Y, Yang Z, Ou C. Zhou H, et al. Bioact Mater. 2024 Dec 3;45:401-416. doi: 10.1016/j.bioactmat.2024.11.016. eCollection 2025 Mar. Bioact Mater. 2024. PMID: 39697241 Free PMC article. - Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation.
Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Liu Y, et al. Am J Physiol Gastrointest Liver Physiol. 2010 Nov;299(5):G1087-96. doi: 10.1152/ajpgi.00124.2010. Epub 2010 Aug 26. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20798357 Free PMC article. - Bioprocessing strategies for enhanced probiotic extracellular vesicle production: culture condition modulation.
Lei Q, Divakarla SK, Winsley T, Roux S, Chrzanowski W. Lei Q, et al. Front Bioeng Biotechnol. 2024 Aug 30;12:1441552. doi: 10.3389/fbioe.2024.1441552. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39280339 Free PMC article. - Cooperation of liver cells in health and disease.
Kmieć Z. Kmieć Z. Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review. - Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body.
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Cristofori F, et al. Front Immunol. 2021 Feb 26;12:578386. doi: 10.3389/fimmu.2021.578386. eCollection 2021. Front Immunol. 2021. PMID: 33717063 Free PMC article. Review.
Cited by
- Recombinant Limosilactobacillus (Lactobacillus) delivering nanobodies against Clostridium perfringens NetB and alpha toxin confers potential protection from necrotic enteritis.
Gangaiah D, Ryan V, Van Hoesel D, Mane SP, Mckinley ET, Lakshmanan N, Reddy ND, Dolk E, Kumar A. Gangaiah D, et al. Microbiologyopen. 2022 Apr;11(2):e1270. doi: 10.1002/mbo3.1270. Microbiologyopen. 2022. PMID: 35478283 Free PMC article. - Targeting Helicobacter pylori Through the "Muco-Microbiotic Layer" Lens: The Challenge of Probiotics and Microbiota Nanovesicles.
Manna OM, Caruso Bavisotto C, Gratie MI, Damiani P, Bonaventura G, Cappello F, Tomasello G, D'Andrea V. Manna OM, et al. Nutrients. 2025 Feb 3;17(3):569. doi: 10.3390/nu17030569. Nutrients. 2025. PMID: 39940427 Free PMC article. Review. - Proteomic and metabolomic profiling of extracellular vesicles produced by human gut archaea.
Weinberger V, Darnhofer B, Thapa HB, Mertelj P, Stentz R, Jones E, Grabmann G, Mohammadzadeh R, Shinde T, Karner C, Ober J, Juodeikis R, Pernitsch D, Hingerl K, Zurabishvili T, Kumpitsch C, Kuehnast T, Rinner B, Strohmaier H, Kolb D, Gotts K, Weichhart T, Köcher T, Köfeler H, Carding SR, Schild S, Moissl-Eichinger C. Weinberger V, et al. Nat Commun. 2025 Jun 3;16(1):5094. doi: 10.1038/s41467-025-60271-w. Nat Commun. 2025. PMID: 40461460 Free PMC article. - The activation impact of lactobacillus-derived extracellular vesicles on lipopolysaccharide-induced microglial cell.
Yang Y, Li N, Gao Y, Xu F, Chen H, Zhang C, Ni X. Yang Y, et al. BMC Microbiol. 2024 Feb 28;24(1):70. doi: 10.1186/s12866-024-03217-4. BMC Microbiol. 2024. PMID: 38418961 Free PMC article. - Roles of bacterial extracellular vesicles in systemic diseases.
Wang Y, Luo X, Xiang X, Hao C, Ma D. Wang Y, et al. Front Microbiol. 2023 Sep 28;14:1258860. doi: 10.3389/fmicb.2023.1258860. eCollection 2023. Front Microbiol. 2023. PMID: 37840728 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources