BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting - PubMed (original) (raw)
BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting
Noa Dagan et al. N Engl J Med. 2021.
Abstract
Background: As mass vaccination campaigns against coronavirus disease 2019 (Covid-19) commence worldwide, vaccine effectiveness needs to be assessed for a range of outcomes across diverse populations in a noncontrolled setting. In this study, data from Israel's largest health care organization were used to evaluate the effectiveness of the BNT162b2 mRNA vaccine.
Methods: All persons who were newly vaccinated during the period from December 20, 2020, to February 1, 2021, were matched to unvaccinated controls in a 1:1 ratio according to demographic and clinical characteristics. Study outcomes included documented infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), symptomatic Covid-19, Covid-19-related hospitalization, severe illness, and death. We estimated vaccine effectiveness for each outcome as one minus the risk ratio, using the Kaplan-Meier estimator.
Results: Each study group included 596,618 persons. Estimated vaccine effectiveness for the study outcomes at days 14 through 20 after the first dose and at 7 or more days after the second dose was as follows: for documented infection, 46% (95% confidence interval [CI], 40 to 51) and 92% (95% CI, 88 to 95); for symptomatic Covid-19, 57% (95% CI, 50 to 63) and 94% (95% CI, 87 to 98); for hospitalization, 74% (95% CI, 56 to 86) and 87% (95% CI, 55 to 100); and for severe disease, 62% (95% CI, 39 to 80) and 92% (95% CI, 75 to 100), respectively. Estimated effectiveness in preventing death from Covid-19 was 72% (95% CI, 19 to 100) for days 14 through 20 after the first dose. Estimated effectiveness in specific subpopulations assessed for documented infection and symptomatic Covid-19 was consistent across age groups, with potentially slightly lower effectiveness in persons with multiple coexisting conditions.
Conclusions: This study in a nationwide mass vaccination setting suggests that the BNT162b2 mRNA vaccine is effective for a wide range of Covid-19-related outcomes, a finding consistent with that of the randomized trial.
Copyright © 2021 Massachusetts Medical Society.
Figures
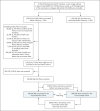
Figure 1. Study Population and Cohort Enrollment Process, December 20, 2020, to February 1, 2021.
The 1,503,216 persons vaccinated before February 1, 2021, were also required to be without a documented SARS-CoV-2 PCR-positive result before the vaccination date. Absolute numbers and percentage changes are shown for each inclusion and exclusion criterion. The exclusion process was gradual and occurred in phases; persons could have had more than one reason for exclusion. The same exclusion criteria were applied to the unvaccinated persons for each index date in which they were considered for matching. The chart focuses on the vaccinated population. CHS denotes Clalit Health Services.
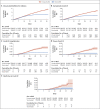
Figure 2. Cumulative Incidence of the Five Outcomes.
Cumulative incidence curves (1 minus the Kaplan–Meier risk) for the various outcomes are shown, starting from the day of administration of the first dose of vaccine. Shaded areas represent 95% confidence intervals. The number at risk at each time point and the cumulative number of events are also shown for each outcome. Graphs in which all data are shown with a y axis scale from 0 to 100 (along with the data shown, as here, on an expanded y axis) are provided in Figure S8 in the Supplementary Appendix.
Comment in
- Blunting COVID-19's negative impact: Lessons from Israel's vaccination campaign.
Tsigaris P, Teixeira da Silva JA. Tsigaris P, et al. Travel Med Infect Dis. 2021 May-Jun;41:102029. doi: 10.1016/j.tmaid.2021.102029. Epub 2021 Mar 16. Travel Med Infect Dis. 2021. PMID: 33737163 Free PMC article. No abstract available. - BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting.
Roest S, Hoek RAS, Manintveld OC. Roest S, et al. N Engl J Med. 2021 May 20;384(20):1968-1970. doi: 10.1056/NEJMc2104281. Epub 2021 Apr 21. N Engl J Med. 2021. PMID: 33882226 No abstract available. - Vaccinating People with Obesity for COVID-19: EASO Call for Action.
Dicker D, Golan R, Baker JL, Busetto L, Frühbeck G, Goossens GH, Halford JCG, Holm JC, Woodward E, Farpour-Lambert NJ. Dicker D, et al. Obes Facts. 2021;14(3):334-335. doi: 10.1159/000516524. Epub 2021 Apr 29. Obes Facts. 2021. PMID: 33915546 Free PMC article. No abstract available. - Impact of first dose of BNT162b2 vaccine on COVID-19 infection among healthcare workers in an Irish hospital.
Walsh J, Skally M, Traynor L, de Barra E, Dhuthaigh AN, Hayes B, Fitzpatrick F. Walsh J, et al. Ir J Med Sci. 2022 Apr;191(2):961-962. doi: 10.1007/s11845-021-02658-4. Epub 2021 May 27. Ir J Med Sci. 2022. PMID: 34041693 Free PMC article. No abstract available. - Letter from Israel.
Berkman N. Berkman N. Respirology. 2022 Jan;27(1):88-89. doi: 10.1111/resp.14180. Epub 2021 Nov 1. Respirology. 2022. PMID: 34725895 No abstract available.
Similar articles
- Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data.
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Haas EJ, et al. Lancet. 2021 May 15;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8. Epub 2021 May 5. Lancet. 2021. PMID: 33964222 Free PMC article. - Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans.
Dickerman BA, Gerlovin H, Madenci AL, Kurgansky KE, Ferolito BR, Figueroa Muñiz MJ, Gagnon DR, Gaziano JM, Cho K, Casas JP, Hernán MA. Dickerman BA, et al. N Engl J Med. 2022 Jan 13;386(2):105-115. doi: 10.1056/NEJMoa2115463. Epub 2021 Dec 1. N Engl J Med. 2022. PMID: 34942066 Free PMC article. - Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting.
Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Barda N, et al. N Engl J Med. 2021 Sep 16;385(12):1078-1090. doi: 10.1056/NEJMoa2110475. Epub 2021 Aug 25. N Engl J Med. 2021. PMID: 34432976 Free PMC article. - Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study.
Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Barda N, et al. Lancet. 2021 Dec 4;398(10316):2093-2100. doi: 10.1016/S0140-6736(21)02249-2. Epub 2021 Oct 29. Lancet. 2021. PMID: 34756184 Free PMC article. - Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women.
Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, Segal Y. Goldshtein I, et al. JAMA. 2021 Aug 24;326(8):728-735. doi: 10.1001/jama.2021.11035. JAMA. 2021. PMID: 34251417 Free PMC article.
Cited by
- The BNT162b2 vaccine is associated with lower new COVID-19 cases in nursing home residents and staff.
Domi M, Leitson M, Gifford D, Nicolaou A, Sreenivas K, Bishnoi C. Domi M, et al. J Am Geriatr Soc. 2021 Aug;69(8):2079-2089. doi: 10.1111/jgs.17224. Epub 2021 May 18. J Am Geriatr Soc. 2021. PMID: 33955567 Free PMC article. - Disease Severity and Durability of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Response: A View Through the Lens of the Second Year of the Pandemic.
Pirofski LA. Pirofski LA. Clin Infect Dis. 2021 Sep 15;73(6):e1345-e1347. doi: 10.1093/cid/ciab374. Clin Infect Dis. 2021. PMID: 33905478 Free PMC article. No abstract available. - The Limited Effect of a History of COVID-19 on Antibody Titers and Adverse Reactions Following BNT162b2 Vaccination: A Single-Center Prospective Study.
Kushima H, Ishii H, Kinoshita Y, Koide Y, Komiya Y, Kato J, Umehara M, Sakata M, Miyazaki M, Ikuta M. Kushima H, et al. J Clin Med. 2022 Sep 14;11(18):5388. doi: 10.3390/jcm11185388. J Clin Med. 2022. PMID: 36143040 Free PMC article. - Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods.
Ribeiro TB, Roque F, Ida F, Plácido AI, Vu M, Hernández-Muñoz JJ, Herdeiro MT. Ribeiro TB, et al. Vaccines (Basel). 2022 Nov 10;10(11):1896. doi: 10.3390/vaccines10111896. Vaccines (Basel). 2022. PMID: 36366404 Free PMC article. - Saliva and Plasma Neutralizing Activity Induced by the Administration of a Third bnt162b2 Vaccine Dose.
Garziano M, Utyro O, Strizzi S, Vanetti C, Saulle I, Conforti C, Cicilano F, Ardizzone F, Cappelletti G, Clerici M, Limanaqi F, Biasin M. Garziano M, et al. Int J Mol Sci. 2022 Nov 18;23(22):14341. doi: 10.3390/ijms232214341. Int J Mol Sci. 2022. PMID: 36430815 Free PMC article.
References
- ClinicalTrials.gov. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. 2020. (https://clinicaltrials.gov/ct2/show/NCT04368728).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous