Exercise improves the quality of slow-wave sleep by increasing slow-wave stability - PubMed (original) (raw)
Randomized Controlled Trial
. 2021 Feb 24;11(1):4410.
doi: 10.1038/s41598-021-83817-6.
Javier Díaz # 1, Sumire Matsumoto 1, Kaito Iwayama 2, Yoshiharu Nabekura 3, Hitomi Ogata 4, Momoko Kayaba 5, Atsushi Aoyagi 3, Katsuhiko Yajima 6, Makoto Satoh 1, Kumpei Tokuyama 1, Kaspar E Vogt 7
Affiliations
- PMID: 33627708
- PMCID: PMC7904822
- DOI: 10.1038/s41598-021-83817-6
Randomized Controlled Trial
Exercise improves the quality of slow-wave sleep by increasing slow-wave stability
Insung Park et al. Sci Rep. 2021.
Abstract
Exercise can improve sleep by reducing sleep latency and increasing slow-wave sleep (SWS). Some studies, however, report adverse effects of exercise on sleep architecture, possibly due to a wide variety of experimental conditions used. We examined the effect of exercise on quality of sleep using standardized exercise parameters and novel analytical methods. In a cross-over intervention study we examined the effect of 60 min of vigorous exercise at 60% [Formula: see text]max on the metabolic state, assessed by core body temperature and indirect calorimetry, and on sleep quality during subsequent sleep, assessed by self-reported quality of sleep and polysomnography. In a novel approach, envelope analysis was performed to assess SWS stability. Exercise increased energy expenditure throughout the following sleep phase. The subjective assessment of sleep quality was not improved by exercise. Polysomnography revealed a shorter rapid eye movement latency and reduced time spent in SWS. Detailed analysis of the sleep electro-encephalogram showed significantly increased delta power in SWS (N3) together with increased SWS stability in early sleep phases, based on delta wave envelope analysis. Although vigorous exercise does not lead to a subjective improvement in sleep quality, sleep function is improved on the basis of its effect on objective EEG parameters.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
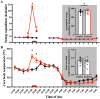
Study protocol. The schedule of the control day (upper bar) and exercise day (bottom bar). For participants whose habitual bedtime is at 00:00, indirect calorimetry begins at 11:00 and ends at 08:00 of the next morning, as shown by the dotted rectangles. Participants exited the metabolic chamber at 19:00 for preparation of the polysomnographic measurement and reentered at 21:00. Gray, red, and white boxes represent sleep (00:00–08:00), exercise (17:00–18:00), and wakefulness (08:00–24:00), respectively. Breakfast, lunch, and dinner are denoted by B, L, and D, respectively.
Figure 2
Time-course of energy expenditure and core body temperature. Time-course of energy expenditure (A) and core body temperature (B) during the entire experiment is shown. Hourly means ± SE are shown for control (filled black circle) and exercise trials (filled red circle), respectively. The red bar at the bottom represents exercise or a sedentary period, and the gray area represents the sleep period. To attach PSG electrodes, participants exited from the metabolic chamber (19:00–21:00). *Represents a statistically significant difference between control and exercise trials by post hoc comparisons using Bonferroni’s correction for multiple comparisons (*p < 0.05).
Figure 3
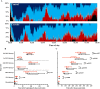
Time-course of sleep architecture and timing of sleep cycles. (A) Sleep architecture of the 9 participants for the control (upper panel) and exercise trials (bottom panel). Percentage of participants in stage W (wakefulness; black), stage N1 (gray), stage N2 (light blue), SWS (dark blue), and stage REM (red) changed with the sleep time. B and C: Latencies of SWS and REM sleep evaluated as time after beginning of sleep cycle (B) and as time after sleep onset (C) are shown. Latency of sleep stage transition in each sleep cycle is shown with black and red box-whisker plots for control and exercise trials, respectively. * and † represent statistically significant differences between the control trial and exercise trial by a paired t-test (*p < 0.05; †p < 0.1).
Figure 4
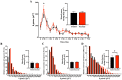
Time-Course of δ-Power of the non-REM Sleep EEG & Relative Occurrence of δ-Power in Each non-REM stage. (A) The 30-min means ± SE of δ-power of the 9 participants are shown as a line graph and accumulated δ-power during non-REM is shown as a bar graph. *Represents a statistically significant difference between the control trial and exercise trial by post hoc comparisons using Bonferroni’s correction for multiple comparisons (p < 0.05). (B–D) Relative occurrences of δ-power in N1 (B), N2 (C), and SWS (D) stages are shown. Inserted bar graphs in each panel represent mean δ-power in each non-REM stage. Black plots (filled black circle) and bars (filled black square) represent control trials, and red plots (filled red circle) and bars (filled red square) represent exercise trials. §Represents a statistically significant difference between control trial and exercise trial by a paired t-test (§p < 0.05).
Figure 5
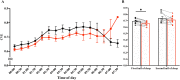
Envelope analysis. (A) Time-course of the CVE during the entire sleep. The 30-min means ± SE of the CVE are shown for the control trial (filled black circle) and exercise trial (filled red circle). (B) Mean CVE during the first half and second half of sleep are shown. Mean CVE is shown for the control trial (open black square) and exercise trial (open red square). Dotted lines connect the same participants. *Represents a significant difference between the control trial and exercise trial by a paired t-test (*p < 0.05). Note that the CVE values did not differ significantly between control and exercise in the last hour of sleep. CVE values were most likely affected by the very low δ power values during this time.
Similar articles
- Cell-Type-Specific Dynamics of Calcium Activity in Cortical Circuits over the Course of Slow-Wave Sleep and Rapid Eye Movement Sleep.
Niethard N, Brodt S, Born J. Niethard N, et al. J Neurosci. 2021 May 12;41(19):4212-4222. doi: 10.1523/JNEUROSCI.1957-20.2021. Epub 2021 Apr 8. J Neurosci. 2021. PMID: 33833082 Free PMC article. - Selective slow-wave sleep suppression affects glucose tolerance and melatonin secretion. The role of sleep architecture.
Ukraintseva YV, Liaukovich KM, Saltykov KA, Belov DA, Nizhnik АN. Ukraintseva YV, et al. Sleep Med. 2020 Mar;67:171-183. doi: 10.1016/j.sleep.2019.11.1254. Epub 2019 Dec 17. Sleep Med. 2020. PMID: 31935619 - Quantitative electroencephalography measures in rapid eye movement and nonrapid eye movement sleep are associated with apnea-hypopnea index and nocturnal hypoxemia in men.
Appleton SL, Vakulin A, D'Rozario A, Vincent AD, Teare A, Martin SA, Wittert GA, McEvoy RD, Catcheside PG, Adams RJ. Appleton SL, et al. Sleep. 2019 Jul 8;42(7):zsz092. doi: 10.1093/sleep/zsz092. Sleep. 2019. PMID: 31004167 - Slow-wave sleep: From the cell to the clinic.
Léger D, Debellemaniere E, Rabat A, Bayon V, Benchenane K, Chennaoui M. Léger D, et al. Sleep Med Rev. 2018 Oct;41:113-132. doi: 10.1016/j.smrv.2018.01.008. Epub 2018 Feb 5. Sleep Med Rev. 2018. PMID: 29490885 Review. - Disorders of Arousal and timing of the first period of slow wave sleep: Clinical and forensic implications.
Pressman MR. Pressman MR. Sleep Med X. 2022 Sep 21;4:100057. doi: 10.1016/j.sleepx.2022.100057. eCollection 2022 Dec. Sleep Med X. 2022. PMID: 36187082 Free PMC article. Review.
Cited by
- Sleep and physical activity - the dynamics of bi-directional influences over a fortnight.
Pesonen AK, Kahn M, Kuula L, Korhonen T, Leinonen L, Martinmäki K, Gradisar M, Lipsanen J. Pesonen AK, et al. BMC Public Health. 2022 Jun 10;22(1):1160. doi: 10.1186/s12889-022-13586-y. BMC Public Health. 2022. PMID: 35681198 Free PMC article. - Bidirectional associations between physical activity and sleep in older adults: a multilevel analysis using polysomnography.
Seol J, Lee J, Park I, Tokuyama K, Fukusumi S, Kokubo T, Yanagisawa M, Okura T. Seol J, et al. Sci Rep. 2022 Sep 13;12(1):15399. doi: 10.1038/s41598-022-19841-x. Sci Rep. 2022. PMID: 36100642 Free PMC article. - On the Road to Camarón: The Sleep of an Ultra-Endurance Athlete Cycling 10,000 km in 24 Days.
Nédélec M, Chauvineau M, Guilhem G. Nédélec M, et al. Int J Environ Res Public Health. 2022 Apr 9;19(8):4543. doi: 10.3390/ijerph19084543. Int J Environ Res Public Health. 2022. PMID: 35457410 Free PMC article. - The causality between leisure sedentary behaviors, physical activity and obstructive sleep apnea: a bidirectional Mendelian randomization study.
Tian H, Wang A, Wu H, Zhou C, Zhang Z, Wang J. Tian H, et al. Front Public Health. 2024 Jun 21;12:1425060. doi: 10.3389/fpubh.2024.1425060. eCollection 2024. Front Public Health. 2024. PMID: 38975351 Free PMC article. - Stable Expression of dmiR-283 in the Brain Promises Positive Effects in Endurance Exercise on Sleep-Wake Behavior in Aging Drosophila.
Li Q, Wang L, Cao Y, Wang X, Tang C, Zheng L. Li Q, et al. Int J Mol Sci. 2023 Feb 20;24(4):4180. doi: 10.3390/ijms24044180. Int J Mol Sci. 2023. PMID: 36835595 Free PMC article.
References
- Sivertsen, B., Krokstad, S., Øverland, S. & Mykletun, A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J. Psychosom. Res.67, 109–116. 10.1016/j.jpsychores.2009.05.001. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical