Understanding the Basis of METH Mouth Using a Rodent Model of Methamphetamine Injection, Sugar Consumption, and Streptococcus mutans Infection - PubMed (original) (raw)
Understanding the Basis of METH Mouth Using a Rodent Model of Methamphetamine Injection, Sugar Consumption, and Streptococcus mutans Infection
Hiu Ham Lee et al. mBio. 2021.
Abstract
"METH mouth" is a common consequence of chronic methamphetamine (METH) use, resulting in tooth decay and painful oral tissue inflammation that can progress to complete tooth loss. METH reduces the amount of saliva in the mouth, promoting bacterial growth, tooth decay, and oral tissue damage. This oral condition is worsened by METH users' compulsive behavior, including high rates of consumption of sugary drinks, recurrent tooth grinding, and a lack of frequent oral hygiene. Streptococcus mutans is a Gram-positive bacterium found in the oral cavity and associated with caries in humans. Hence, we developed a murine model of METH administration, sugar intake, and S. mutans infection to mimic METH mouth in humans and to investigate the impact of this drug on tooth colonization. We demonstrated that the combination of METH and sucrose stimulates S. mutans tooth adhesion, growth, and biofilm formation in vivo METH and sucrose increased the expression of S. mutans glycosyltransferases and lactic acid production. Moreover, METH contributes to the low environmental pH and S. mutans sucrose metabolism, providing a plausible mechanism for bacterium-mediated tooth decay. Daily oral rinse treatment with chlorhexidine significantly reduces tooth colonization in METH- and sucrose-treated mice. Furthermore, human saliva inhibits S. mutans colonization and biofilm formation after exposure to either sucrose or the combination of METH and sucrose. These findings suggest that METH might increase the risk of microbial dental disease in users, information that may help in the development of effective public health strategies to deal with this scourge in our society.IMPORTANCE "METH mouth" is characterized by severe tooth decay and gum disease, which often causes teeth to break or fall out. METH users are also prone to colonization by cariogenic bacteria such as Streptococcus mutans In addition, this oral condition is aggravated by METH users' compulsive behavior, including the consumption of beverages with high sugar content, recurrent tooth grinding, and a lack of frequent oral hygiene. We investigated the effects of METH and sugar consumption on S. mutans biofilm formation and tooth colonization. Using a murine model of METH administration, sucrose ingestion, and oral infection, we found that the combination of METH and sucrose increases S. mutans adhesion and biofilm formation on the teeth of C57BL/6 mice. However, daily chlorhexidine-based oral rinse treatment reduces S. mutans tooth colonization. Similarly, METH has been associated with dry mouth or hyposalivation in users. Hence, we assessed the impact of human saliva on biofilm formation and demonstrated that surface preconditioning with saliva substantially reduces S. mutans biofilm formation. Our results are significant because to our knowledge, this is the first basic science study focused on elucidating the fundamentals of METH mouth using a rodent model of prolonged drug injection and S. mutans oral infection. Our findings may have important translational implications for the development of treatments for the management of METH mouth and more effective preventive public health strategies that can be applied to provide effective dental care for METH users in prisons, drug treatment centers, and health clinics.
Keywords: METH mouth; Streptococcus mutans; biofilms; chlorhexidine; methamphetamine; sucrose.
Copyright © 2021 Lee et al.
Figures
FIG 1
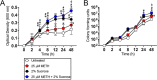
Methamphetamine (METH) and sucrose stimulate S. mutans replication in vitro. The effect of METH, sucrose, or their combination on S. mutans growth kinetics was determined via Bioscreen C (A) and CFU (B) analyses. S. mutans was grown in the absence (untreated) or presence of 25 μM METH, 2% sucrose, or 25 μM METH plus 2% sucrose. For real-time spectrophotometry and cell viability assays, each time point represents the average from 16 and 5 individual measurements, respectively. Symbols (*, #, and ϕ) indicate higher proliferation rates than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. These experiments were performed twice, with similar results obtained each time.
FIG 2
The combination of METH and sucrose promotes adherence of viable Streptococcus mutans to a plastic surface after 4 h of incubation. Bacteria were grown in the absence (untreated) or presence of 25 μM METH, 2% sucrose, and 25 μM METH plus 2% sucrose for 4 h at 37°C in a 5% CO2 aerobic atmosphere. Next, S. mutans adhesion to the polystyrene substrate was determined by fluorescence microscopy (A), measurements of bacterial adhesion per field (n = 10 replicates under each condition) (B), and CFU counts (n = 6 replicates under each condition) (C). For panels B and C, violin plots denote the averages (dashed lines) and replicate distributions. Each assay was performed twice independently, and all the replicates were included in the graphs. Symbols (*, #, and ϕ) indicate high statistical significance compared to the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of the Bonferroni correction. These assays were carried out in triplicate and performed twice, with similar results obtained each time.
FIG 3
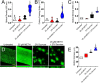
The combination of METH and sucrose enhances S. mutans biofilm formation in vitro. (A to C) S. mutans biofilm formation on polystyrene microtiter plates was evaluated by an XTT reduction assay (A), CFU determinations (B), and crystal violet staining (C) after incubation of the bacteria with phosphate-buffered saline (PBS) (untreated), 25 μM METH, 2% sucrose, and 25 μM METH plus 2% sucrose for 48 h at 37°C in a 5% CO2 aerobic atmosphere. Violin plots denote the averages (dashed lines) and replicate distributions (n = 8 under each condition). All these assays were carried out in quadruplicates and performed twice independently, and all the replicates were included in the graphs. (D) Confocal microscopy of mature S. mutans biofilms formed on glass-bottom plates after incubation of the bacteria (green [SYTO 9]) alone (PBS) (untreated) or with 25 μM METH, 2% sucrose, and 25 μM METH plus 2% sucrose for 48 h at 37°C. The pictures were taken at a magnification of ×63. Bars, 100 μm. (E) The thickness of the bacterial biofilms grown under these conditions was measured by _z_-stack reconstruction. Violin plots show the averages (dashed lines) and distributions from three independent thickness measurements. For panels A to C and E, symbols (*, #, and ϕ) indicate higher statistical significance than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test.
FIG 4
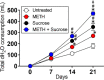
The combination of METH and sucrose increases water (H2O) consumption by C57BL/6 mice. The animals’ total H2O consumption after 21 days of METH injection and sucrose administration is shown. Mice were daily injected intraperitoneally with PBS (untreated) or METH (2.5, 5, and 10 mg/kg/day on weeks 1, 2, and 3, respectively). In addition, two groups of mice, PBS and METH treated, were supplemented with 2% sucrose in the drinking H2O. H2O consumption by rodents (n = 5 per cage per group) was monitored and recorded every 7 days during 21 days of treatments. Each time point represents three independent measurements. Symbols (*, #, and ϕ) indicate higher H2O consumption rates than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. These experiments were performed twice, with similar results obtained each time.
FIG 5
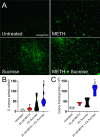
The combination of METH and sucrose increases S. mutans biofilm formation on the teeth of C57BL/6 mice. Shown are scanning electron microscopy (SEM) images of mature S. mutans biofilms formed on the teeth of C57BL/6 mice for 24 h. After METH and sucrose treatments, mice were infected orally with 107S. mutans bacteria and sacrificed after 24 h, and their frontal teeth were carefully extracted for imaging (bar, 20 μm) (A), crystal violet staining (B), and CFU determinations (C). For panels B and C, violin plots signify the averages (dashed lines) and subject distributions (n = 10 for crystal violet staining and n = 6 for CFU determinations per group) under each experimental condition. Each assay was performed twice independently, and all the animals for each experiment were included in the graphs. Symbols (*, #, and ϕ) indicate higher statistical significance than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. a.u., arbitrary units.
FIG 6
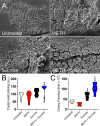
The combination of METH and sucrose promotes S. mutans tooth adhesion in C57BL/6 mice. (A) SEM images of streptococcal cells adhered on the teeth of C57BL/6 mice after 4 h of oral infection with 107 bacteria. Bars, 10 μm and 1 mm (inset). (B) Attachment of bacteria to the teeth of untreated animals or animals treated with METH, 2% sucrose, and METH plus 2% sucrose after 4 h of oral infection was evaluated by counting CFU. Violin plots indicate the averages (dashed lines) and distributions of the results for five animals per group. Symbols (*, #, and ϕ) indicate higher adhesion than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test.
FIG 7
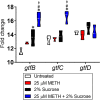
The combination of METH and sucrose induces the expression of the S. mutans glucosyltransferase genes gtfB and gtfC. The differential expression of S. mutans glucosyltransferase-encoding genes (gtfB, gtfC, and gtfD) was measured using reverse transcriptase PCR. Bacteria were cultured in the absence or presence of 25 μM METH, 2% sucrose, or the combination for 24 h. 16S rRNA was used as the housekeeping gene control. Violin plots represent the averages and distributions from three independent measurements in triplicates. Symbols (*, #, and ϕ) indicate significantly higher expression levels than in the untreated, 25 μM METH, or 2% sucrose group, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test.
FIG 8
Sucrose promotes S. mutans environmental pH reduction. (A) Changes in brain heart infusion (BHI) broth pH by S. mutans were measured using a pH meter. The following conditions were tested: BHI broth alone or supplemented with 25 μM METH, 2% sucrose, or METH plus 2% sucrose in the absence or presence of S. mutans. Violin plots indicate the averages (dashed lines) and distributions from eight independent measurements. Each symbol (ε, &, δ, ψ, σ, %, χ, and ω) denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. ε, &, δ, ψ, σ, and % indicate significantly lower pH than in the BHI broth, BHI broth plus 25 μM METH, BHI broth plus 2% sucrose, BHI broth plus 25 μM METH and 2% sucrose, BHI broth plus S. mutans, and BHI broth plus 25 μM METH and S. mutans groups, respectively. χ and ω denote significantly higher pH than in the BHI broth and BHI broth plus 25 μM METH groups, respectively. (B) Lactic acid production by S. mutans was quantified after incubation in the absence (untreated) or presence of 25 μM METH, 2% sucrose, or METH plus 2% sucrose. Violin plots indicate the averages (dashed lines) and distributions from four measurements. Each symbol (*, ϕ, and %) denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. * and ϕ indicate significantly higher lactic acid production than in the untreated and 2% sucrose groups, respectively. % denotes significantly lower lactic acid synthesis than in the 25 μM METH group. Both assays were performed twice independently, and all the replicates are shown in each graph.
FIG 9
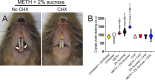
Daily mouth rinse of C57BL/6 mice infected with S. mutans reduces bacterial tooth colonization. (A) Photographs of C57BL/6 mice injected with METH, supplemented with 2% sucrose in the drinking H2O, infected with 107S. mutans, and treated daily with either PBS (no chlorhexidine [CHX]) or CHX for 7 days. White arrows denote crystal violet staining (purple) as indicative of S. mutans tooth colonization and biofilm formation. (B) Crystal violet staining was used to quantify the biofilm biomass on the teeth of mice at 7 days postinfection. The following groups were tested: untreated and uninfected, untreated, METH, 2% sucrose, METH plus 2% sucrose, CHX, METH plus CHX, 2% sucrose plus CHX, and METH plus 2% sucrose and CHX. Violin plots indicate the averages (dashed lines) and distributions from 10 independent measurements (2 teeth per mouse; n = 5). Symbols (ε, *, #, ϕ, $, @, λ, and π) indicate significantly higher staining than in the untreated and uninfected, untreated, METH, 2% sucrose, CHX, METH plus CHX, 2% sucrose plus CHX, and METH plus 2% sucrose and CHX groups, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test.
FIG 10
Surface preconditioning with human saliva considerably reduces S. mutans biofilm formation in vitro. (A to C) Biofilm formation on 96-well microtiter plates was determined by an XTT reduction assay (A), CFU determination (B), and crystal violet staining (C) after 1 h of preconditioning of the plastic surface with 100 μl of PBS or saliva and incubation of bacteria with PBS (untreated), 25 μM METH, 2% sucrose, and 25 μM METH plus 2% sucrose for 48 h at 37°C in a 5% CO2 aerobic atmosphere. Violin plots indicate the averages (dashed lines) and replicate distributions (n = 8 under each condition). All these assays were carried out in quadruplicates under each condition and performed twice independently, and all replicates were included in each graph. Symbols for the saline (*, #, and ϕ) and saliva (@, &, and %) conditions indicate significantly higher values than in the untreated, METH, 2% sucrose, and METH plus 2% sucrose groups, respectively. Each symbol denotes P value significance (P < 0.05) calculated by ANOVA and adjusted by the use of Tukey’s multiple-comparison test. Crosses (saline versus saliva) indicate P value significance (P < 0.05) calculated using Student’s t test. (D) Confocal microscopy of mature S. mutans biofilms formed on preconditioned glass-bottom plates with saline or saliva after incubation of the bacteria (green [SYTO 9]) with 25 μM METH plus 2% sucrose for 48 h at 37°C. The pictures were taken at a magnification of ×63. Bars, 100 μm. (E) The thickness of the streptococcal biofilms grown under these conditions was measured by _z_-stack reconstruction. Violin plots represent the averages and distributions from three independent measurements. The sigma symbol denotes P value significance (P < 0.05) calculated by Student’s t test.
Similar articles
- Influence of sucrose and xylitol on an early Streptococcus mutans biofilm in a dental simulator.
Salli KM, Forssten SD, Lahtinen SJ, Ouwehand AC. Salli KM, et al. Arch Oral Biol. 2016 Oct;70:39-46. doi: 10.1016/j.archoralbio.2016.05.020. Epub 2016 Jun 6. Arch Oral Biol. 2016. PMID: 27318453 - Dietary sugars modulate bacterial-fungal interactions in saliva and inter-kingdom biofilm formation on apatitic surface.
Negrini TC, Ren Z, Miao Y, Kim D, Simon-Soro Á, Liu Y, Koo H, Arthur RA. Negrini TC, et al. Front Cell Infect Microbiol. 2022 Nov 9;12:993640. doi: 10.3389/fcimb.2022.993640. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439211 Free PMC article. - Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis.
Díaz-Garrido N, Lozano CP, Kreth J, Giacaman RA. Díaz-Garrido N, et al. Appl Environ Microbiol. 2020 Oct 15;86(21):e01262-20. doi: 10.1128/AEM.01262-20. Print 2020 Oct 15. Appl Environ Microbiol. 2020. PMID: 32826216 Free PMC article. - The virulence of Streptococcus mutans and the ability to form biofilms.
Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. Krzyściak W, et al. Eur J Clin Microbiol Infect Dis. 2014 Apr;33(4):499-515. doi: 10.1007/s10096-013-1993-7. Epub 2013 Oct 24. Eur J Clin Microbiol Infect Dis. 2014. PMID: 24154653 Free PMC article. Review. - Does assessment of microbial composition of plaque/saliva allow for diagnosis of disease activity of individuals?
Bowden GH. Bowden GH. Community Dent Oral Epidemiol. 1997 Feb;25(1):76-81. doi: 10.1111/j.1600-0528.1997.tb00902.x. Community Dent Oral Epidemiol. 1997. PMID: 9088695 Review.
Cited by
- Exploring the Interplay Between Lifestyle Medicine and Oral Health: A Bidirectional Relationship.
Ciantelli NMM, Yoong J, Deschamps J, Jaqua EE. Ciantelli NMM, et al. Am J Lifestyle Med. 2023 Nov 7;18(3):425-430. doi: 10.1177/15598276231213339. eCollection 2024 May-Jun. Am J Lifestyle Med. 2023. PMID: 38737885 Free PMC article. - The Effects of Drug Addiction and Detoxification on the Human Oral Microbiota.
Zhang J, Liu W, Shi L, Liu X, Wang M, Li W, Yu D, Wang Y, Zhang J, Yun K, Yan J. Zhang J, et al. Microbiol Spectr. 2023 Feb 1;11(2):e0396122. doi: 10.1128/spectrum.03961-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36722952 Free PMC article. - The niche-specialist and age-related oral microbial ecosystem: crosstalk with host immune cells in homeostasis.
Lin D, Hu Q, Yang L, Zeng X, Xiao Y, Wang D, Dai W, Lu H, Fang J, Tang Z, Wang Z. Lin D, et al. Microb Genom. 2022 Jun;8(6):mgen000811. doi: 10.1099/mgen.0.000811. Microb Genom. 2022. PMID: 35731208 Free PMC article. - How Do Drugs Affect the Skeleton? Implications for Forensic Anthropology.
Márquez-Grant N, Baldini E, Jeynes V, Biehler-Gomez L, Aoukhiyad L, Passalacqua NV, Giordano G, Di Candia D, Cattaneo C. Márquez-Grant N, et al. Biology (Basel). 2022 Mar 29;11(4):524. doi: 10.3390/biology11040524. Biology (Basel). 2022. PMID: 35453723 Free PMC article.
References
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. 2009. The costs of methamphetamine use: a national estimate. Rand Corporation, Santa Monica, CA. 10.7249/RB9438. - DOI
- National Institute on Drug Abuse. 2020. Methamphetamine research report: overview. National Institute on Drug Abuse, Bethesda, MD. https://www.drugabuse.gov/publications/research-reports/methamphetamine/.... Accessed 20 October 2020.
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. 2002. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci 22:2135–2141. doi:10.1523/JNEUROSCI.22-06-02135.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical