N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2 - PubMed (original) (raw)
. 2021 Apr 29;184(9):2332-2347.e16.
doi: 10.1016/j.cell.2021.03.028. Epub 2021 Mar 16.
Anna De Marco 2, Florian A Lempp 3, M Alejandra Tortorici 4, Dora Pinto 2, Alexandra C Walls 1, Martina Beltramello 2, Alex Chen 3, Zhuoming Liu 5, Fabrizia Zatta 2, Samantha Zepeda 1, Julia di Iulio 3, John E Bowen 1, Martin Montiel-Ruiz 3, Jiayi Zhou 3, Laura E Rosen 3, Siro Bianchi 2, Barbara Guarino 2, Chiara Silacci Fregni 2, Rana Abdelnabi 6, Shi-Yan Caroline Foo 6, Paul W Rothlauf 5, Louis-Marie Bloyet 5, Fabio Benigni 2, Elisabetta Cameroni 2, Johan Neyts 6, Agostino Riva 7, Gyorgy Snell 3, Amalio Telenti 3, Sean P J Whelan 5, Herbert W Virgin 3, Davide Corti 8, Matteo Samuele Pizzuto 9, David Veesler 10
Affiliations
- PMID: 33761326
- PMCID: PMC7962585
- DOI: 10.1016/j.cell.2021.03.028
N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2
Matthew McCallum et al. Cell. 2021.
Abstract
The SARS-CoV-2 spike (S) glycoprotein contains an immunodominant receptor-binding domain (RBD) targeted by most neutralizing antibodies (Abs) in COVID-19 patient plasma. Little is known about neutralizing Abs binding to epitopes outside the RBD and their contribution to protection. Here, we describe 41 human monoclonal Abs (mAbs) derived from memory B cells, which recognize the SARS-CoV-2 S N-terminal domain (NTD) and show that a subset of them neutralize SARS-CoV-2 ultrapotently. We define an antigenic map of the SARS-CoV-2 NTD and identify a supersite (designated site i) recognized by all known NTD-specific neutralizing mAbs. These mAbs inhibit cell-to-cell fusion, activate effector functions, and protect Syrian hamsters from SARS-CoV-2 challenge, albeit selecting escape mutants in some animals. Indeed, several SARS-CoV-2 variants, including the B.1.1.7, B.1.351, and P.1 lineages, harbor frequent mutations within the NTD supersite, suggesting ongoing selective pressure and the importance of NTD-specific neutralizing mAbs for protective immunity and vaccine design.
Keywords: COVID-19; N-terminal domain; NTD; SARS-CoV-2; memory B cells; neutralizing antibody; spike glycoprotein.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.D.M., F.A.L., D.P., M.B., F.Z., J.d.I., M.M.-R., J.Z., L.E.R., S.B., B.G., C.S.F., F.B., E.C., G.S., A.T., H.W.V., D.C., and M.S.P. are employees of Vir Biotechnology Inc. and may hold shares in Vir Biotechnology Inc. D.C. is currently listed as an inventor on multiple patent applications, which disclose the subject matter described in this manuscript. The Neyts laboratories have received sponsored research agreements from Vir Biotechnology Inc. H.W.V. is a founder of PierianDx and Casma Therapeutics. Neither company provided funding for this work or is performing related work. D.V. is a consultant for Vir Biotechnology Inc. The Veesler laboratory has received a sponsored research agreement from Vir Biotechnology Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
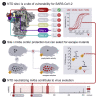
Graphical abstract
Figure 1
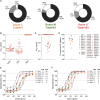
Discovery of potent NTD-specific SARS-CoV-2 neutralizing mAbs from three convalescent individuals (A) Pie charts showing the frequency of mAbs (cloned from IgG+ memory B cells) recognizing the SARS-CoV-2 NTD, RBD, or other S regions for patients L, M, and X. (B) Binding of the 41 isolated NTD mAbs to immobilized SARS-CoV-2 S, NTD, or RBD analyzed by ELISA. (C and D) Neutralization potencies (IC50, C) and maximal neutralization plateau (NT, D) of 15 NTD-specific neutralizing mAbs against SARS-CoV-2 S MLV pseudotyped virus. Data are from one out of two independent experiments performed. (E and F) Dose-dependent neutralization of selected NTD- and RBD-specific mAbs against authentic SARS-CoV-2-Nluc assessed 6 h (at MOI 0.1) (E) or 24 h (at MOI of 0.01) (F) after infection. One independent experiment out of at least two is shown. Error bars indicate standard deviation of triplicates. See also Figure S1, Table S1, and Data S1.
Figure S1
Characteristics of SARS-CoV-2 NTD mAbs related to Figure 1 (A) Biolayer interferometry binding kinetic analysis of the SARS-CoV-2 NTD to immobilized mAbs. (B and C) V gene usage for the heavy (B) and light (C) chains of the NTD mAbs. (D) Nucleotide sequence identity of the mAbs isolated relative to the respective V germline genes. (E) HCDR3 amino acid length for individual mAbs. (F) Cell-to-cell fusion inhibition assay with Vero E6 cells transfected with SARS-CoV-2 S and incubated with varying concentrations of S2L28, S2M28, S2X28, S2X333 or the RBD-specific mAb S2M11. (G) Binding of NTD- and RBD-specific mAbs to immobilized SARS-CoV-2 S at pH7 and pH5 as analyzed by ELISA. One independent experiment out of two is shown.
Figure 2
SARS-CoV-2 NTD neutralizing mAbs target the same antigenic supersite (A–C) Ribbon diagrams in two orthogonal orientations of the SARS-CoV-2 S ectodomain trimer (surface) bound to the RBD-specific S2M11 Fab (gray) and to the NTD-targeted Fab S2L28 (A), S2M28 (B), or S2X333 (C). (D–F) S2L28 (D), S2M28 (E), and SX333 (F) binding pose relative to the NTD. (G–I) Zoomed-in views showing selected interactions of S2L28 (G), S2M28 (H), or S2X333 (I) with the NTD. SARS-CoV-2 S protomers are colored pink, cyan, and gold whereas N-linked glycans are rendered as dark blue surfaces. See also Figure S2 and Tables S2–S4.
Figure S2
Cryo-EM data processing of SARS-CoV-2 S bound to S2L28, S2M28, or S2X333, related to Figure 2 (Top) Unsharpened maps colored by local resolution calculated using cryoSPARC for the S trimer bound to S2M11 Fab and either S2L28 (A), S2M28 (B), or S2X333 Fab (C), as well as the locally refined reconstruction of NTD-bound Fab (inset). (Bottom) Representative electron micrograph and class averages (bottom left of each panel) are shown for SARS-CoV-2 S in complex with the indicated Fabs embedded in vitreous ice (Scale bar: 100 nm).
Figure S3
Cryo-EM data processing of SARS-CoV-2 S bound to S2X28, S2L20, S2X316, or S2M24, related to Figures 2 and 3 (A) 6 Å low-pass filtered map of the SARS-CoV-2 2P DS S trimer (McCallum et al., 2020) bound to S2X28 (gold). (B–D) Sharpened maps of the S trimer bound to S2M11 Fabs and S2L20 (B, gray), S2X316 (C, purple), or S2M24 Fabs (D, sky blue). Representative electron micrographs and class averages are shown at the bottom left of each panel (Scale bar: 100 nm). The corresponding Fourier shell correlation curves (bottom right of each panel) are shown with the 0.143 cutoff indicated by horizontal dashed lines.
Figure 3
The SARS-CoV-2 NTD comprises multiple antigenic sites (A) Epitope binning of the 41 NTD-specific mAbs isolated led to the identification of six antigenic sites based on competition binding assays using biolayer interferometry. The competition assays were performed either using the S ectodomain trimer or the NTD (NTD). NC, no competition; PC, partial competition; C, competition; ∗, potently neutralizing mAbs. One independent experiment out of two is shown. (B and C) Composite model of the SARS-CoV-2 S trimer viewed along two orthogonal orientations with four bound Fab fragments representative of antigenic site i (S2X333), site iv (S2L20), site v (S2X316), and site vi (S2M24). (D) Footprints of the antigenic sites identified structurally are shown along with neighboring N-linked glycans (blue spheres). See also Figure S3, Table S5, and Data S1.
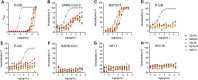
Figure 4
NTD mAbs are affected by the genetic diversity of circulating SARS-CoV-2 variants and sarbecoviruses (A) Prevalence of SARS-CoV-2 S variants among circulating isolates as of February 12, 2021 (508,771 sequences). The NTD and RBD are highlighted with red and gray backgrounds, respectively. The NTD supersite residues are highlighted by darker red background shading. (B) S2L28, S2M28, S2X28, S2X333, 4A8, and S2L20 binding to recombinant SARS-CoV-2 NTD variants analyzed by ELISA and displayed as a heatmap. 69/70del, deletion of residues 69/70; Y144del, deletion of residue Y144. EC50s were calculated as mean of duplicates from two technical replicates. (C) Heatmap of NTD mAb binding to a panel of sarbecovirus S glycoproteins expressed at the surface of ExpiCHO cells analyzed by flow cytometry. Binding is expressed as mean fluorescence intensities relative to SARS-CoV-2 S binding for each mAb. One independent experiment out of two is shown. A coronavirus NTD cladogram is shown on the left. The conservation of relevant glycans and NTD supersite regions is also shown (right). See also Figure S4 and Data S1.
Figure S4
NTD neutralizing mAbs inhibit S-mediated entry of the closely related RaTG13 but not of more distant sarbecoviruses, related to Figure 4 (A) Binding of NTD- and RBD-specific mAbs to immobilized P-GD S ectodomain trimer analyzed by ELISA. (B–H) VSV pseudovirus neutralization assays in the presence of varying concentrations of the NTD-specific mAbs S2L28, S2M28, S2X58, S2X333 or the RBD-specific mAb S2E12.
Figure 5
Analysis of SARS-CoV-2 S neutralization escape mutants (A) Escape mutations selected with each mAb (black background white font) and residues that remained unchanged (white background) upon passaging a VSV-SARS-CoV-2 S chimeric virus. (B) S2L28, S2M28, S2X28, S2X333, 4A8, and S2L20 binding to selected SARS-CoV-2 NTD escape mutants analyzed by ELISA and displayed as a heatmap. The native signal peptide was introduced using the wild-type sequence (SP) or with the S12P mutation (SP+S12P). EC50s were calculated as mean of duplicates from two technical replicates. (C–F) Deconvoluted mass spectra of purified NTD constructs, including the NTD construct with an optimized signal peptide (C, NTD), the NTD construct with an optimized signal peptide and the C136Y mutation (D, NTD), the NTD construct with the native signal peptide (E, NTD), and the NTD construct with the native signal peptide carrying the S12P mutation (F, NTD). The empirical mass (black) and theoretical mass (red) are shown beside the corresponding peak. Additional 119 Da were observed for the C136Y and SP+S12P NTDs corresponding to cysteinylation of the free cysteine residue in these constructs (as L-cysteine was present in the expression media). The cleaved signal peptide (black text white background) and subsequent N-terminal sequence (white text black background) are also shown based on the mass spectrometry (MS) results; C15 is highlighted in light red. Peptides identified by MS/MS analysis consistent with the mass of N-terminal peptides are shown above the N-terminal sequence (black horizontal lines). See also Data S1.
Figure 6
Mechanism of action of NTD-specific neutralizing mAbs (A) Competition of S2L28, S2M28, S2X28, and S2X333 with ACE2 to bind to SARS-CoV-2 S as measured by biolayer interferometry. ACE2 was immobilized at the surface of the biosensors before incubation with the S ectodomain trimer alone or pre-complexed with mAbs. The vertical dashed line indicates the start of the association of free S or S/mAb complexes with solid-phase ACE2. The anti-RBD S2E12 mAb was included as positive control. (B) mAb-mediated S1 subunit shedding from cell surface expressed SARS-CoV-2 S as determined by flow cytometry. The anti-RBD S2M11 and S2E12 mAbs were included as negative and positive controls, respectively. (C) Neutralization of authentic SARS-CoV-2 (SARS-CoV-2-Nluc) by S2L28, S2M28, S2X28, and S2X333 IgG or Fab. S309 and S2M11 Fab and IgG were also included as controls. Symbols are means ± SD of triplicates. Dotted lines indicate IC50 and IC90 values. (D) SPR analysis of mAbs binding to the SARS-CoV-2 S ectodomain trimer. Gray dashed line indicates a fit to a 1:1 binding model. The equilibrium dissociation constants (KD) or apparent equilibrium dissociation constants (KD, app) are indicated. White and gray stripes indicate association and dissociation phases, respectively. (E and F) Activation of FcγRIIa H131 (E) and FcγRIIIa V158 (F) induced by NTD-specific mAbs. The anti-RBD mAb S309 was included as positive control. One independent experiment out of at least two is shown. See also Figures S1 and S5 and Table S1.
Figure S5
Neutralizing activity of SARS-CoV-2 NTD- and RBD-targeting mAb cocktails, related to Figures 1 and 6 (A–C) SARS-CoV-2-MLV pseudotypes neutralization (left) and synergy score (right) measured combining S2X333 with the RBD-targeting mAb S309 (A), S2E12 (B), or S2M11 (C). (D) Neutralization matrix to assess the synergistic activity of S2X333 and S309 mAb cocktails in vitro with authentic SARS-CoV-2-Nluc. Data for authentic SARS-CoV-2-Nluc are from one representative experiment performed in triplicate each.
Figure 7
S2X333 provides robust in vivo protection against SARS-CoV-2 challenge but selects for escape mutants Syrian hamsters were injected with the indicated amount of mAb 48 h before intra-nasal challenge with SARS-CoV-2. (A) Quantification of viral RNA in the lungs 4 days post-infection. (B) Quantification of replicating virus in lung homogenates harvested 4 days post-infection using a TCID50 assay. (C and D) Viral RNA loads and replicating virus titers in the lung 4 days post-infection plotted as a function of serum mAb concentrations before infection (day 0). Mann-Whitney test was used. ∗p < 0.05, ∗∗p < 0.01. Data from two independent experiment are presented. (Irrelevant mAb n = 8; S2X333 1 mg/kg n = 9; S2X333 4 mg/kg n = 9). See also Figure S6.
Figure S6
Sequence alignment of SARS-CoV-2 S vRNA from input virus, control group, and selected outlier animals administered with S2X333, related to Figure 7 Nucleotide alignment of a region of interest reveals a deletion encompassing amino acid at position 144 only in S2X333-treated animals. SARS-CoV-2 S Wuhan = reference sequence (NCBI Reference Sequence NC_045512.2); SARS-CoV-2 S -p6-20200525 = virus stock; hamster 172 = animal from the control group; hamster 394 = outlier (TCID50) from the group administered with S2X333 at 1 mg/kg; hamster 397 = outlier (TCID50) from the group administered with S2X333 at 4 mg/kg. Alignment was performed using CLC Main Workbench 21 (QIAGEN)
Update of
- N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2.
McCallum M, Marco A, Lempp F, Tortorici MA, Pinto D, Walls AC, Beltramello M, Chen A, Liu Z, Zatta F, Zepeda S, di Iulio J, Bowen JE, Montiel-Ruiz M, Zhou J, Rosen LE, Bianchi S, Guarino B, Fregni CS, Abdelnabi R, Caroline Foo SY, Rothlauf PW, Bloyet LM, Benigni F, Cameroni E, Neyts J, Riva A, Snell G, Telenti A, Whelan SPJ, Virgin HW, Corti D, Pizzuto MS, Veesler D. McCallum M, et al. bioRxiv [Preprint]. 2021 Jan 14:2021.01.14.426475. doi: 10.1101/2021.01.14.426475. bioRxiv. 2021. PMID: 33469588 Free PMC article. Updated. Preprint.
Comment in
- An NTD supersite of attack.
Lok SM. Lok SM. Cell Host Microbe. 2021 May 12;29(5):744-746. doi: 10.1016/j.chom.2021.04.010. Cell Host Microbe. 2021. PMID: 33984277 Free PMC article.
Similar articles
- N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2.
McCallum M, Marco A, Lempp F, Tortorici MA, Pinto D, Walls AC, Beltramello M, Chen A, Liu Z, Zatta F, Zepeda S, di Iulio J, Bowen JE, Montiel-Ruiz M, Zhou J, Rosen LE, Bianchi S, Guarino B, Fregni CS, Abdelnabi R, Caroline Foo SY, Rothlauf PW, Bloyet LM, Benigni F, Cameroni E, Neyts J, Riva A, Snell G, Telenti A, Whelan SPJ, Virgin HW, Corti D, Pizzuto MS, Veesler D. McCallum M, et al. bioRxiv [Preprint]. 2021 Jan 14:2021.01.14.426475. doi: 10.1101/2021.01.14.426475. bioRxiv. 2021. PMID: 33469588 Free PMC article. Updated. Preprint. - A Combination of Receptor-Binding Domain and N-Terminal Domain Neutralizing Antibodies Limits the Generation of SARS-CoV-2 Spike Neutralization-Escape Mutants.
Haslwanter D, Dieterle ME, Wec AZ, O'Brien CM, Sakharkar M, Florez C, Tong K, Rappazzo CG, Lasso G, Vergnolle O, Wirchnianski AS, Bortz RH 3rd, Laudermilch E, Fels JM, Mengotto A, Malonis RJ, Georgiev GI, Quiroz JA, Wrapp D, Wang N, Dye KE, Barnhill J, Dye JM, McLellan JS, Daily JP, Lai JR, Herbert AS, Walker LM, Chandran K, Jangra RK. Haslwanter D, et al. mBio. 2021 Oct 26;12(5):e0247321. doi: 10.1128/mBio.02473-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607456 Free PMC article. - Cross-Neutralization of Emerging SARS-CoV-2 Variants of Concern by Antibodies Targeting Distinct Epitopes on Spike.
Changrob S, Fu Y, Guthmiller JJ, Halfmann PJ, Li L, Stamper CT, Dugan HL, Accola M, Rehrauer W, Zheng NY, Huang M, Wang J, Erickson SA, Utset HA, Graves HM, Amanat F, Sather DN, Krammer F, Kawaoka Y, Wilson PC. Changrob S, et al. mBio. 2021 Dec 21;12(6):e0297521. doi: 10.1128/mBio.02975-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781736 Free PMC article. Clinical Trial. - Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.
Hussain A, Hasan A, Nejadi Babadaei MM, Bloukh SH, Chowdhury MEH, Sharifi M, Haghighat S, Falahati M. Hussain A, et al. Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review. - Is SARS-CoV-2 Spike Evolution Being Retargeted at the N-Terminal Domain?
Focosi D, Alfonsi T, Bernasconi A. Focosi D, et al. Discov Med. 2025 May;37(196):801-807. doi: 10.24976/Discov.Med.202537196.70. Discov Med. 2025. PMID: 40415355 Review.
Cited by
- Accumulation of mutations in antibody and CD8 T cell epitopes in a B cell depleted lymphoma patient with chronic SARS-CoV-2 infection.
Khatamzas E, Antwerpen MH, Rehn A, Graf A, Hellmuth JC, Hollaus A, Mohr AW, Gaitzsch E, Weiglein T, Georgi E, Scherer C, Stecher SS, Gruetzner S, Blum H, Krebs S, Reischer A, Leutbecher A, Subklewe M, Dick A, Zange S, Girl P, Müller K, Weigert O, Hopfner KP, Stemmler HJ, von Bergwelt-Baildon M, Keppler OT, Wölfel R, Muenchhoff M, Moosmann A. Khatamzas E, et al. Nat Commun. 2022 Sep 23;13(1):5586. doi: 10.1038/s41467-022-32772-5. Nat Commun. 2022. PMID: 36151076 Free PMC article. - Implemented occupational health surveillance limits the spread of SARS-CoV-2 Omicron at the workplace.
Gesto JSM, Cabanelas A, Farjun B, Dos Santos MC, Fidalgo-Neto AA, Kuriyama SN, Souza TML. Gesto JSM, et al. Front Med (Lausanne). 2022 Aug 30;9:910176. doi: 10.3389/fmed.2022.910176. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36111122 Free PMC article. - Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses.
Chen Y, Zhao X, Zhou H, Zhu H, Jiang S, Wang P. Chen Y, et al. Nat Rev Immunol. 2023 Mar;23(3):189-199. doi: 10.1038/s41577-022-00784-3. Epub 2022 Sep 27. Nat Rev Immunol. 2023. PMID: 36168054 Free PMC article. Review. - Differential laboratory passaging of SARS-CoV-2 viral stocks impacts the in vitro assessment of neutralizing antibodies.
Avila-Herrera A, Kimbrel JA, Manuel Martí J, Thissen J, Saada EA, Weisenberger T, Arrildt KT, Segelke BW, Allen JE, Zemla A, Borucki MK. Avila-Herrera A, et al. PLoS One. 2024 Jan 25;19(1):e0289198. doi: 10.1371/journal.pone.0289198. eCollection 2024. PLoS One. 2024. PMID: 38271318 Free PMC article. - Deep mutational scans of XBB.1.5 and BQ.1.1 reveal ongoing epistatic drift during SARS-CoV-2 evolution.
Taylor AL, Starr TN. Taylor AL, et al. PLoS Pathog. 2023 Dec 29;19(12):e1011901. doi: 10.1371/journal.ppat.1011901. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38157379 Free PMC article.
References
- Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM120553/GM/NIGMS NIH HHS/United States
- DP1 AI158186/AI/NIAID NIH HHS/United States
- S10 OD023476/OD/NIH HHS/United States
- HHSN272201700059C/AI/NIAID NIH HHS/United States
- T32 GM008268/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous