The PRISMA 2020 statement: an updated guideline for reporting systematic reviews - PubMed (original) (raw)
doi: 10.1186/s13643-021-01626-4.
Joanne E McKenzie 2, Patrick M Bossuyt 3, Isabelle Boutron 4, Tammy C Hoffmann 5, Cynthia D Mulrow 6, Larissa Shamseer 7, Jennifer M Tetzlaff 8, Elie A Akl 9, Sue E Brennan 2, Roger Chou 10, Julie Glanville 11, Jeremy M Grimshaw 12, Asbjørn Hróbjartsson 13, Manoj M Lalu 14, Tianjing Li 15, Elizabeth W Loder 16, Evan Mayo-Wilson 17, Steve McDonald 2, Luke A McGuinness 18, Lesley A Stewart 19, James Thomas 20, Andrea C Tricco 21, Vivian A Welch 22, Penny Whiting 18, David Moher 23
Affiliations
- PMID: 33781348
- PMCID: PMC8008539
- DOI: 10.1186/s13643-021-01626-4
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
Matthew J Page et al. Syst Rev. 2021.
No abstract available
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/ and declare: EL is head of research for the BMJ; MJP is an editorial board member for PLOS Medicine; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews. None of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health, for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration, which produces systematic reviews, and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocates for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners, creator of DistillerSR software for systematic reviews; Evidence Partners was not involved in the design or outcomes of the statement, and the views expressed solely represent those of the author.
Figures
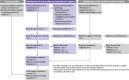
Fig. 1
PRISMA 2020 flow diagram template for systematic reviews. The new design is adapted from flow diagrams proposed by Boers [55], Mayo-Wilson et al. [56] and Stovold et al. [57] The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information
Similar articles
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. Rev Esp Cardiol (Engl Ed). 2021 Sep;74(9):790-799. doi: 10.1016/j.rec.2021.07.010. Rev Esp Cardiol (Engl Ed). 2021. PMID: 34446261 English, Spanish. - The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. Int J Surg. 2021 Apr;88:105906. doi: 10.1016/j.ijsu.2021.105906. Epub 2021 Mar 29. Int J Surg. 2021. PMID: 33789826 - Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration.
Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, Tugwell P; PRISMA-Equity Bellagio group. Welch V, et al. J Clin Epidemiol. 2016 Feb;70:68-89. doi: 10.1016/j.jclinepi.2015.09.001. Epub 2015 Sep 5. J Clin Epidemiol. 2016. PMID: 26348799 - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. J Clin Epidemiol. 2009. PMID: 19631507 - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. Ann Intern Med. 2009 Aug 18;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136. Epub 2009 Jul 20. Ann Intern Med. 2009. PMID: 19622512
Cited by
- Comparative effectiveness of exercise interventions for primary dysmenorrhea: a systematic review and network meta-analysis.
Zheng Q, Huang G, Cao W, Zhao Y. Zheng Q, et al. BMC Womens Health. 2024 Nov 16;24(1):610. doi: 10.1186/s12905-024-03453-w. BMC Womens Health. 2024. PMID: 39550537 Free PMC article. - Skin cancer in patients who are co-infected with HIV/ HBV or HIV/HCV: a systematic review.
Lee W, Cho DK, Ragi SD, Khachemoune A. Lee W, et al. Arch Dermatol Res. 2024 Nov 15;317(1):20. doi: 10.1007/s00403-024-03529-5. Arch Dermatol Res. 2024. PMID: 39546015 Review. - Osteoarthritis and Neurological Disorder Diagnoses in Adults: A Meta-Analysis Examining Associations With Parkinson's Disease, Multiple Sclerosis, and Alzheimer's Disease.
Peoples BM, Harrison KD, Renfrow G, Bethea D, Santamaria Guzman KG, Wilson AE, Samaan MA, Roper JA. Peoples BM, et al. Cureus. 2024 Oct 14;16(10):e71458. doi: 10.7759/cureus.71458. eCollection 2024 Oct. Cureus. 2024. PMID: 39544560 Free PMC article. Review. - Efficacy of Aerobic and Stretching Exercises in Managing Willis-Ekbom Disease (Restless Leg Syndrome) Among Hemodialysis Patients.
Altayb Ismail MA, Daffalla I, Singh T, Siddique QR, Almadhoun MKIK, Irfan R, Saqib M, Haris M, Khan Z, Fernandes JGF, Iqbal A, Bokhari SFH. Altayb Ismail MA, et al. Cureus. 2024 Oct 14;16(10):e71470. doi: 10.7759/cureus.71470. eCollection 2024 Oct. Cureus. 2024. PMID: 39544546 Free PMC article. Review. - Adapted approaches to initial fluid management of patients with major burns in resource-limited settings: A systematic review.
Hsiao KH, Kalanzi J, Watson SB, Murthy S, Movsisyan A, Kothari K, Salio F, Relan P. Hsiao KH, et al. Burns Open. 2024 Nov;8(4):None. doi: 10.1016/j.burnso.2024.100365. Burns Open. 2024. PMID: 39540031 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources