PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews - PubMed (original) (raw)
Guideline
doi: 10.1136/bmj.n160.
David Moher 2, Patrick M Bossuyt 3, Isabelle Boutron 4, Tammy C Hoffmann 5, Cynthia D Mulrow 6, Larissa Shamseer 7, Jennifer M Tetzlaff 8, Elie A Akl 9, Sue E Brennan 10, Roger Chou 11, Julie Glanville 12, Jeremy M Grimshaw 13, Asbjørn Hróbjartsson 14, Manoj M Lalu 15, Tianjing Li 16, Elizabeth W Loder 17, Evan Mayo-Wilson 18, Steve McDonald 10, Luke A McGuinness 19, Lesley A Stewart 20, James Thomas 21, Andrea C Tricco 22, Vivian A Welch 23, Penny Whiting 19, Joanne E McKenzie 10
Affiliations
- PMID: 33781993
- PMCID: PMC8005925
- DOI: 10.1136/bmj.n160
Guideline
PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews
Matthew J Page et al. BMJ. 2021.
Abstract
The methods and results of systematic reviews should be reported in sufficient detail to allow users to assess the trustworthiness and applicability of the review findings. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement was developed to facilitate transparent and complete reporting of systematic reviews and has been updated (to PRISMA 2020) to reflect recent advances in systematic review methodology and terminology. Here, we present the explanation and elaboration paper for PRISMA 2020, where we explain why reporting of each item is recommended, present bullet points that detail the reporting recommendations, and present examples from published reviews. We hope that changes to the content and structure of PRISMA 2020 will facilitate uptake of the guideline and lead to more transparent, complete, and accurate reporting of systematic reviews.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/ and declare: EL is head of research for the BMJ; MJP is an editorial board member for PLOS Medicine; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews; none of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health, for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration which produces systematic reviews and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocate for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners Inc, creators of DistillerSR software for systematic reviews; Evidence Partners Inc was not involved in the design or outcomes of the statement and the views expressed solely represent those of the author.
Figures
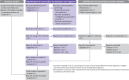
Fig 1
PRISMA 2020 flow diagram template for systematic reviews (adapted from flow diagrams proposed by Boers and Mayo-Wilson et al. and Stovold et al.132). The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
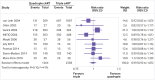
Fig 2
The figure displays for each study included in the meta-analysis the summary statistics (number of events and sample size) for the quadruple and triple combination antiretroviral therapies (cART) groups, and the risk ratio and its 95% confidence interval for the dichotomous outcome, undetectable HIV-1 RNA. Reproduced from Feng et al.
Fig 3
The figure displays for each study included in the meta-analysis the summary statistics (mean, standard deviation, and sample size) for the quadruple and triple combination antiretroviral therapies (cART) groups, and the mean difference and its 95% confidence interval for the continuous outcome, CD4 T cell count (cells/μL). Reproduced from Feng et al.
Similar articles
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. BMJ. 2021. PMID: 33782057 Free PMC article. - The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. J Clin Epidemiol. 2021 Jun;134:178-189. doi: 10.1016/j.jclinepi.2021.03.001. Epub 2021 Mar 29. J Clin Epidemiol. 2021. PMID: 33789819 - Methodological quality of systematic reviews comprising clinical practice guidelines for cardiovascular risk assessment and management for noncardiac surgery.
Jacobsen SM, Douglas A, Smith CA, Roberts W, Ottwell R, Oglesby B, Yasler C, Torgerson T, Hartwell M, Vassar M. Jacobsen SM, et al. Br J Anaesth. 2021 Dec;127(6):905-916. doi: 10.1016/j.bja.2021.08.016. Epub 2021 Sep 20. Br J Anaesth. 2021. PMID: 34548174 - PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews.
Arya S, Kaji AH, Boermeester MA. Arya S, et al. JAMA Surg. 2021 Aug 1;156(8):789-790. doi: 10.1001/jamasurg.2021.0546. JAMA Surg. 2021. PMID: 33825806 No abstract available. - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. J Clin Epidemiol. 2009. PMID: 19631507
Cited by
- Postnatal Development of the Circadian Rhythmicity of Human Pineal Melatonin Synthesis and Secretion (Systematic Review).
Paditz E. Paditz E. Children (Basel). 2024 Sep 29;11(10):1197. doi: 10.3390/children11101197. Children (Basel). 2024. PMID: 39457162 Free PMC article. Review. - The relationship between metabolic syndrome and survival of patients with endometrial cancer: a meta-analysis.
Deng F, Chen Y, Wu Y, Tang Y, Yi W. Deng F, et al. Front Oncol. 2024 Oct 21;14:1484109. doi: 10.3389/fonc.2024.1484109. eCollection 2024. Front Oncol. 2024. PMID: 39497714 Free PMC article. - Barriers and facilitators of collaboration during the implementation of vocational rehabilitation interventions: a systematic review.
Noteboom Y, Montanus AWA, van Nassau F, Burchell G, Anema JR, Huysmans MA. Noteboom Y, et al. BMC Psychiatry. 2024 Nov 1;24(1):759. doi: 10.1186/s12888-024-06223-y. BMC Psychiatry. 2024. PMID: 39487467 Free PMC article. - Probiotics, a promising therapy to reduce the recurrence of bacterial vaginosis in women? a systematic review and meta-analysis of randomized controlled trials.
Chieng WK, Abdul Jalal MI, Bedi JS, Zainuddin AA, Mokhtar MH, Abu MA, Chew KT, Nur Azurah AG. Chieng WK, et al. Front Nutr. 2022 Sep 20;9:938838. doi: 10.3389/fnut.2022.938838. eCollection 2022. Front Nutr. 2022. PMID: 36204368 Free PMC article. - Social prescribing for children and young people with neurodisability and their families initiated in a hospital setting: a systematic review.
Gordon K, Gordon L, Basu AP. Gordon K, et al. BMJ Open. 2023 Dec 21;13(12):e078097. doi: 10.1136/bmjopen-2023-078097. BMJ Open. 2023. PMID: 38135327 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources