The PRISMA 2020 statement: an updated guideline for reporting systematic reviews - PubMed (original) (raw)
Joanne E McKenzie 2, Patrick M Bossuyt 3, Isabelle Boutron 4, Tammy C Hoffmann 5, Cynthia D Mulrow 6, Larissa Shamseer 7, Jennifer M Tetzlaff 8, Elie A Akl 9, Sue E Brennan 2, Roger Chou 10, Julie Glanville 11, Jeremy M Grimshaw 12, Asbjørn Hróbjartsson 13, Manoj M Lalu 14, Tianjing Li 15, Elizabeth W Loder 16, Evan Mayo-Wilson 17, Steve McDonald 2, Luke A McGuinness 18, Lesley A Stewart 19, James Thomas 20, Andrea C Tricco 21, Vivian A Welch 22, Penny Whiting 18, David Moher 23
Affiliations
- PMID: 33782057
- PMCID: PMC8005924
- DOI: 10.1136/bmj.n71
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
Matthew J Page et al. BMJ. 2021.
Abstract
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/ and declare: EL is head of research for the BMJ; MJP is an editorial board member for PLOS Medicine; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews. None of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health, for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration, which produces systematic reviews, and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocates for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners, creator of DistillerSR software for systematic reviews; Evidence Partners was not involved in the design or outcomes of the statement, and the views expressed solely represent those of the author.
Figures
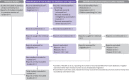
Fig 1
PRISMA 2020 flow diagram template for systematic reviews. The new design is adapted from flow diagrams proposed by Boers, Mayo-Wilson et al. and Stovold et al. The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
Comment in
- Rigour and transparency in the family of systematic reviews: The International Journal of Health Planning and Management encourages prospective protocol registration.
Jesus TS. Jesus TS. Int J Health Plann Manage. 2022 Sep;37(5):2523-2527. doi: 10.1002/hpm.3510. Epub 2022 Jun 7. Int J Health Plann Manage. 2022. PMID: 35672954 No abstract available. - Tissue Sealants for Facial Rhytidectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
Chien WY, Huang YL, Chiu WK, Kang YN, Chen C. Chien WY, et al. Facial Plast Surg Aesthet Med. 2023 Mar-Apr;25(2):90-96. doi: 10.1089/fpsam.2022.0160. Epub 2022 Oct 18. Facial Plast Surg Aesthet Med. 2023. PMID: 36260353 Review. - Efficacy and safety of galcanezumab as a treatment of refractory episodic and chronic cluster headache: Case series and narrative review.
Membrilla JA, Torres-Ferrus M, Alpuente A, Caronna E, Pozo-Rosich P. Membrilla JA, et al. Headache. 2022 Nov;62(10):1395-1405. doi: 10.1111/head.14404. Epub 2022 Nov 2. Headache. 2022. PMID: 36321947 Review.
Similar articles
- PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. Page MJ, et al. BMJ. 2021 Mar 29;372:n160. doi: 10.1136/bmj.n160. BMJ. 2021. PMID: 33781993 Free PMC article. - The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. J Clin Epidemiol. 2021 Jun;134:178-189. doi: 10.1016/j.jclinepi.2021.03.001. Epub 2021 Mar 29. J Clin Epidemiol. 2021. PMID: 33789819 - Methodological quality of systematic reviews comprising clinical practice guidelines for cardiovascular risk assessment and management for noncardiac surgery.
Jacobsen SM, Douglas A, Smith CA, Roberts W, Ottwell R, Oglesby B, Yasler C, Torgerson T, Hartwell M, Vassar M. Jacobsen SM, et al. Br J Anaesth. 2021 Dec;127(6):905-916. doi: 10.1016/j.bja.2021.08.016. Epub 2021 Sep 20. Br J Anaesth. 2021. PMID: 34548174 - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. BMJ. 2009 Jul 21;339:b2700. doi: 10.1136/bmj.b2700. BMJ. 2009. PMID: 19622552 Free PMC article. - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. J Clin Epidemiol. 2009. PMID: 19631507
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous