Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19 - PubMed (original) (raw)
Clinical Trial
. 2021 Jun 10;384(23):2187-2201.
doi: 10.1056/NEJMoa2101544. Epub 2021 Apr 21.
Glenda Gray 1, An Vandebosch 1, Vicky Cárdenas 1, Georgi Shukarev 1, Beatriz Grinsztejn 1, Paul A Goepfert 1, Carla Truyers 1, Hein Fennema 1, Bart Spiessens 1, Kim Offergeld 1, Gert Scheper 1, Kimberly L Taylor 1, Merlin L Robb 1, John Treanor 1, Dan H Barouch 1, Jeffrey Stoddard 1, Martin F Ryser 1, Mary A Marovich 1, Kathleen M Neuzil 1, Lawrence Corey 1, Nancy Cauwenberghs 1, Tamzin Tanner 1, Karin Hardt 1, Javier Ruiz-Guiñazú 1, Mathieu Le Gars 1, Hanneke Schuitemaker 1, Johan Van Hoof 1, Frank Struyf 1, Macaya Douoguih 1; ENSEMBLE Study Group
Collaborators, Affiliations
- PMID: 33882225
- PMCID: PMC8220996
- DOI: 10.1056/NEJMoa2101544
Clinical Trial
Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19
Jerald Sadoff et al. N Engl J Med. 2021.
Abstract
Background: The Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein in a prefusion-stabilized conformation.
Methods: In an international, randomized, double-blind, placebo-controlled, phase 3 trial, we randomly assigned adult participants in a 1:1 ratio to receive a single dose of Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe-critical coronavirus disease 2019 (Covid-19) with an onset at least 14 days and at least 28 days after administration among participants in the per-protocol population who had tested negative for SARS-CoV-2. Safety was also assessed.
Results: The per-protocol population included 19,630 SARS-CoV-2-negative participants who received Ad26.COV2.S and 19,691 who received placebo. Ad26.COV2.S protected against moderate to severe-critical Covid-19 with onset at least 14 days after administration (116 cases in the vaccine group vs. 348 in the placebo group; efficacy, 66.9%; adjusted 95% confidence interval [CI], 59.0 to 73.4) and at least 28 days after administration (66 vs. 193 cases; efficacy, 66.1%; adjusted 95% CI, 55.0 to 74.8). Vaccine efficacy was higher against severe-critical Covid-19 (76.7% [adjusted 95% CI, 54.6 to 89.1] for onset at ≥14 days and 85.4% [adjusted 95% CI, 54.2 to 96.9] for onset at ≥28 days). Despite 86 of 91 cases (94.5%) in South Africa with sequenced virus having the 20H/501Y.V2 variant, vaccine efficacy was 52.0% and 64.0% against moderate to severe-critical Covid-19 with onset at least 14 days and at least 28 days after administration, respectively, and efficacy against severe-critical Covid-19 was 73.1% and 81.7%, respectively. Reactogenicity was higher with Ad26.COV2.S than with placebo but was generally mild to moderate and transient. The incidence of serious adverse events was balanced between the two groups. Three deaths occurred in the vaccine group (none were Covid-19-related), and 16 in the placebo group (5 were Covid-19-related).
Conclusions: A single dose of Ad26.COV2.S protected against symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection and was effective against severe-critical disease, including hospitalization and death. Safety appeared to be similar to that in other phase 3 trials of Covid-19 vaccines. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722.).
Copyright © 2021 Massachusetts Medical Society.
Figures
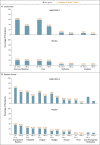
Figure 1. Solicited Local and Systemic Adverse Events Reported within 7 days after the Administration of Vaccine or Placebo (Safety Subpopulation).
Most solicited local and systemic adverse events occurred within 1 to 2 days after the administration of vaccine or placebo and had a median duration of 1 to 2 days. No grade 4 local or systemic adverse events were reported. There were no local or systemic reactogenicity differences between participants who were seronegative at baseline and those who were seropositive (data not shown). Pain was categorized as grade 1 (mild; does not interfere with activity), grade 2 (moderate; requires modification of activity or involves discomfort with movement), grade 3 (severe; inability to perform usual activities), or grade 4 (potentially life-threatening; hospitalization or inability to perform basic self-care). Erythema and swelling were categorized as grade 1 (mild; 25 to 50 mm), grade 2 (moderate; 51 to 100 mm), grade 3 (severe; >100 mm), or grade 4 (potentially life-threatening; necrosis or leading to hospitalization). Systemic events were categorized as grade 1 (mild; minimal symptoms), grade 2 (moderate; notable symptoms not resulting in loss of work or school time), grade 3 (severe; incapacitating symptoms resulting in loss of work or school time), or grade 4 (life-threatening; hospitalization or inability to perform basic self-care). Fever was defined as grade 1 (mild; ≥38.0 to 38.4°C), grade 2 (moderate; ≥38.5 to 38.9°C), grade 3 (severe; ≥39.0 to 40.0°C), or grade 4 (potentially life-threatening; >40°C).
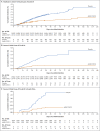
Figure 2. Cumulative Incidence of Covid-19 with Onset at Least 1 Day after Vaccination and Vaccine Efficacy over Time.
Panel A shows the cumulative incidence of moderate to severe–critical cases of coronavirus disease 2019 (Covid-19); circles indicate severe–critical cases. Panel B shows the cumulative incidence of severe–critical cases. Cases included in the analyses in Panels A and B were centrally confirmed cases in the full analysis set among participants who were seronegative at baseline. Panel C shows the cumulative incidence of severe–critical cases in South Africa among participants who were seronegative at baseline; these cases were those that were positive on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing from all sources, whether centrally confirmed or not.
Comment in
- Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19.
Heininger U. Heininger U. N Engl J Med. 2021 Jul 15;385(3):288. doi: 10.1056/NEJMc2107809. Epub 2021 Jun 9. N Engl J Med. 2021. PMID: 34107178 No abstract available. - The single-dose J&J vaccine had 67% efficacy against moderate to severe-critical COVID-19 at ≥14 d.
Sacks HS. Sacks HS. Ann Intern Med. 2021 Jul;174(7):JC75. doi: 10.7326/ACPJ202107200-075. Epub 2021 Jul 6. Ann Intern Med. 2021. PMID: 34224270
Similar articles
- Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Van Dromme I, Spiessens B, Vingerhoets J, Custers J, Scheper G, Robb ML, Treanor J, Ryser MF, Barouch DH, Swann E, Marovich MA, Neuzil KM, Corey L, Stoddard J, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Sadoff J, et al. N Engl J Med. 2022 Mar 3;386(9):847-860. doi: 10.1056/NEJMoa2117608. Epub 2022 Feb 9. N Engl J Med. 2022. PMID: 35139271 Free PMC article. Clinical Trial. - Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial.
Hardt K, Vandebosch A, Sadoff J, Le Gars M, Truyers C, Lowson D, Van Dromme I, Vingerhoets J, Kamphuis T, Scheper G, Ruiz-Guiñazú J, Faust SN, Spinner CD, Schuitemaker H, Van Hoof J, Douoguih M, Struyf F; ENSEMBLE2 study group. Hardt K, et al. Lancet Infect Dis. 2022 Dec;22(12):1703-1715. doi: 10.1016/S1473-3099(22)00506-0. Epub 2022 Sep 13. Lancet Infect Dis. 2022. PMID: 36113538 Free PMC article. Clinical Trial. - Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine.
Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, Berghmans PJ, Kimmel M, Van Damme P, de Hoon J, Smith W, Stephenson KE, De Rosa SC, Cohen KW, McElrath MJ, Cormier E, Scheper G, Barouch DH, Hendriks J, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H. Sadoff J, et al. N Engl J Med. 2021 May 13;384(19):1824-1835. doi: 10.1056/NEJMoa2034201. Epub 2021 Jan 13. N Engl J Med. 2021. PMID: 33440088 Free PMC article. Clinical Trial. - Efficacy and safety of COVID-19 vaccines.
Graña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, Buckley BS, Probyn K, Villanueva G, Henschke N, Bonnet H, Assi R, Menon S, Marti M, Devane D, Mallon P, Lelievre JD, Askie LM, Kredo T, Ferrand G, Davidson M, Riveros C, Tovey D, Meerpohl JJ, Grasselli G, Rada G, Hróbjartsson A, Ravaud P, Chaimani A, Boutron I. Graña C, et al. Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review. - Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.
Le Gars M, Hendriks J, Sadoff J, Ryser M, Struyf F, Douoguih M, Schuitemaker H. Le Gars M, et al. Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
Cited by
- Safety and Humoral Immunogenicity of Different Dose Levels of Ad26.COV2.S as a 2-Dose Regimen in COVID-19 Vaccine-Naïve Healthy Adults: A Phase 3 Randomized Clinical Trial.
Rezelj VV, Paddenburg F, Diegbe ME, Nangosyah J, Reisinger EC, Hu W, Truyers C, Scheper G, Le Gars M, Hendriks J, Struyf F, Douoguih M, Schuitemaker H, Ruiz-Guiñazú J. Rezelj VV, et al. Vaccines (Basel). 2024 Oct 3;12(10):1136. doi: 10.3390/vaccines12101136. Vaccines (Basel). 2024. PMID: 39460303 Free PMC article. - Trends in willingness to receive COVID-19 vaccines among healthcare workers in India: Findings from repeated cross-sectional national surveys.
Padhi BK, Chakrapani V, Gupta M, Sharma N, Patro BK, Kar SS, Singh R, Pala S, Sankhe L, Modi B, Bali S, Rustagi N, Jain L, Vij J, Satapathy P, Goel K, Rajagopal V, Kiran T, Aggarwal AK. Padhi BK, et al. Front Public Health. 2022 Oct 3;10:994206. doi: 10.3389/fpubh.2022.994206. eCollection 2022. Front Public Health. 2022. PMID: 36262227 Free PMC article. - Serum peptidome profiles immune response of COVID-19 Vaccine administration.
Zhang W, Li D, Xu B, Xu L, Lyu Q, Liu X, Li Z, Zhang J, Sun W, Ma Q, Qiao L, Liao P. Zhang W, et al. Front Immunol. 2022 Aug 24;13:956369. doi: 10.3389/fimmu.2022.956369. eCollection 2022. Front Immunol. 2022. PMID: 36091008 Free PMC article. - Low-frequency CD8+ T cells induced by SIGN-R1+ macrophage-targeted vaccine confer SARS-CoV-2 clearance in mice.
Muraoka D, Moi ML, Muto O, Nakatsukasa T, Deng S, Takashima C, Yamaguchi R, Sawada SI, Hayakawa H, Nguyen TTN, Haseda Y, Soga T, Matsushita H, Ikeda H, Akiyoshi K, Harada N. Muraoka D, et al. NPJ Vaccines. 2024 Sep 18;9(1):173. doi: 10.1038/s41541-024-00961-6. NPJ Vaccines. 2024. PMID: 39294173 Free PMC article. - Naturally occurring spike mutations influence the infectivity and immunogenicity of SARS-CoV-2.
Peng Q, Zhou R, Liu N, Wang H, Xu H, Zhao M, Yang D, Au KK, Huang H, Liu L, Chen Z. Peng Q, et al. Cell Mol Immunol. 2022 Nov;19(11):1302-1310. doi: 10.1038/s41423-022-00924-8. Epub 2022 Oct 12. Cell Mol Immunol. 2022. PMID: 36224497 Free PMC article.
References
- Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. (https://coronavirus.jhu.edu/map.html).
- Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. December 22, 2020. (https://www.medrxiv.org/content/10.1101/2020.12.21.20248640v1). preprint.
- Public Health England. Investigation of novel SARS-CoV-2 variant: variant of concern 202012/01: technical briefing 3. 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploa...).
- Krammer F. SARS-CoV-2 vaccines in development. Nature 2020;586:516-527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- U01 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- HHSO100201700018C/AA/NIAAA NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- U01 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- S10 AI174104/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous