COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study - PubMed (original) (raw)
Multicenter Study
. 2021 May 8;397(10286):1725-1735.
doi: 10.1016/S0140-6736(21)00790-X. Epub 2021 Apr 23.
Sarah Foulkes 2, Ayoub Saei 2, Nick Andrews 3, Blanche Oguti 4, Andre Charlett 5, Edgar Wellington 2, Julia Stowe 2, Natalie Gillson 2, Ana Atti 2, Jasmin Islam 2, Ioannis Karagiannis 2, Katie Munro 2, Jameel Khawam 2, Meera A Chand 6, Colin S Brown 2, Mary Ramsay 3, Jamie Lopez-Bernal 2, Susan Hopkins 7; SIREN Study Group
Collaborators, Affiliations
- PMID: 33901423
- PMCID: PMC8064668
- DOI: 10.1016/S0140-6736(21)00790-X
Multicenter Study
COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study
Victoria Jane Hall et al. Lancet. 2021.
Abstract
Background: BNT162b2 mRNA and ChAdOx1 nCOV-19 adenoviral vector vaccines have been rapidly rolled out in the UK from December, 2020. We aimed to determine the factors associated with vaccine coverage for both vaccines and documented the vaccine effectiveness of the BNT162b2 mRNA vaccine in a cohort of health-care workers undergoing regular asymptomatic testing.
Methods: The SIREN study is a prospective cohort study among staff (aged ≥18 years) working in publicly-funded hospitals in the UK. Participants were assigned into either the positive cohort (antibody positive or history of infection [indicated by previous positivity of antibody or PCR tests]) or the negative cohort (antibody negative with no previous positive test) at the beginning of the follow-up period. Baseline risk factors were collected at enrolment, symptom status was collected every 2 weeks, and vaccination status was collected through linkage to the National Immunisations Management System and questionnaires. Participants had fortnightly asymptomatic SARS-CoV-2 PCR testing and monthly antibody testing, and all tests (including symptomatic testing) outside SIREN were captured. Data cutoff for this analysis was Feb 5, 2021. The follow-up period was Dec 7, 2020, to Feb 5, 2021. The primary outcomes were vaccinated participants (binary ever vacinated variable; indicated by at least one vaccine dose recorded by at least one of the two vaccination data sources) for the vaccine coverage analysis and SARS-CoV-2 infection confirmed by a PCR test for the vaccine effectiveness analysis. We did a mixed-effect logistic regression analysis to identify factors associated with vaccine coverage. We used a piecewise exponential hazard mixed-effects model (shared frailty-type model) using a Poisson distribution to calculate hazard ratios to compare time-to-infection in unvaccinated and vaccinated participants and estimate the impact of the BNT162b2 vaccine on all PCR-positive infections (asymptomatic and symptomatic). This study is registered with ISRCTN, number ISRCTN11041050, and is ongoing.
Findings: 23 324 participants from 104 sites (all in England) met the inclusion criteria for this analysis and were enrolled. Included participants had a median age of 46·1 years (IQR 36·0-54·1) and 19 692 (84%) were female; 8203 (35%) were assigned to the positive cohort at the start of the analysis period, and 15 121 (65%) assigned to the negative cohort. Total follow-up time was 2 calendar months and 1 106 905 person-days (396 318 vaccinated and 710 587 unvaccinated). Vaccine coverage was 89% on Feb 5, 2021, 94% of whom had BNT162b2 vaccine. Significantly lower coverage was associated with previous infection, gender, age, ethnicity, job role, and Index of Multiple Deprivation score. During follow-up, there were 977 new infections in the unvaccinated cohort, an incidence density of 14 infections per 10 000 person-days; the vaccinated cohort had 71 new infections 21 days or more after their first dose (incidence density of eight infections per 10 000 person-days) and nine infections 7 days after the second dose (incidence density four infections per 10 000 person-days). In the unvaccinated cohort, 543 (56%) participants had typical COVID-19 symptoms and 140 (14%) were asymptomatic on or 14 days before their PCR positive test date, compared with 29 (36%) with typical COVID-19 symptoms and 15 (19%) asymptomatic in the vaccinated cohort. A single dose of BNT162b2 vaccine showed vaccine effectiveness of 70% (95% CI 55-85) 21 days after first dose and 85% (74-96) 7 days after two doses in the study population.
Interpretation: Our findings show that the BNT162b2 vaccine can prevent both symptomatic and asymptomatic infection in working-age adults. This cohort was vaccinated when the dominant variant in circulation was B1.1.7 and shows effectiveness against this variant.
Funding: Public Health England, UK Department of Health and Social Care, and the National Institute for Health Research.
Crown copyright © 2021 Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no competing interests. The Immunisation and Countermeasures Division at PHE has provided vaccine manufacturers (including Pfizer) with post-marketing surveillance reports on pneumococcal and meningococcal infection which the companies are required to submit to the UK Licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports.
Figures
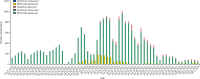
Figure 1
Number of vaccinated SIREN participants by dose, manufacturer, and day, Dec 8, 2020 to Feb 5, 2021 (n=20 641)
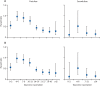
Figure 2
Adjusted hazard ratios at post-vaccination intervals in the (A) full cohort (n=23 324) and (B) negative cohort (n=15 121), Dec 7, 2020 to Feb 5, 2021 Hazard ratios were adjusted for site as a random effect, period, and eight fixed effects: age, gender, ethnicity, comorbidities, job role, frequency of contact with COVID-19 patients, employed in a patient facing role, and occupational exposure.
Comment in
- Population immunity and vaccine protection against infection.
Leshem E, Lopman BA. Leshem E, et al. Lancet. 2021 May 8;397(10286):1685-1687. doi: 10.1016/S0140-6736(21)00870-9. Epub 2021 Apr 23. Lancet. 2021. PMID: 33901422 Free PMC article. No abstract available.
Similar articles
- Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study.
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, Lopez Bernal J, Moss P, Hayward A, Copas A, Shallcross L. Shrotri M, et al. Lancet Infect Dis. 2021 Nov;21(11):1529-1538. doi: 10.1016/S1473-3099(21)00289-9. Epub 2021 Jun 23. Lancet Infect Dis. 2021. PMID: 34174193 Free PMC article. - SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN).
Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, Wellington E, Cole MJ, Saei A, Oguti B, Munro K, Wallace S, Kirwan PD, Shrotri M, Vusirikala A, Rokadiya S, Kall M, Zambon M, Ramsay M, Brooks T, Brown CS, Chand MA, Hopkins S; SIREN Study Group. Hall VJ, et al. Lancet. 2021 Apr 17;397(10283):1459-1469. doi: 10.1016/S0140-6736(21)00675-9. Epub 2021 Apr 9. Lancet. 2021. PMID: 33844963 Free PMC article. - Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers.
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Angel Y, et al. JAMA. 2021 Jun 22;325(24):2457-2465. doi: 10.1001/jama.2021.7152. JAMA. 2021. PMID: 33956048 Free PMC article. - Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data.
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Haas EJ, et al. Lancet. 2021 May 15;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8. Epub 2021 May 5. Lancet. 2021. PMID: 33964222 Free PMC article. - How evolutionary science can help us understand vaccine refusal in the COVID-19 pandemic.
Swanepoel A, Abed R, Kaser M, St John Smith P. Swanepoel A, et al. BJPsych Bull. 2023 Aug;47(4):224-228. doi: 10.1192/bjb.2022.36. BJPsych Bull. 2023. PMID: 35818884 Free PMC article. Review.
Cited by
- Population immunity and vaccine protection against infection.
Leshem E, Lopman BA. Leshem E, et al. Lancet. 2021 May 8;397(10286):1685-1687. doi: 10.1016/S0140-6736(21)00870-9. Epub 2021 Apr 23. Lancet. 2021. PMID: 33901422 Free PMC article. No abstract available. - The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis.
Rahmani K, Shavaleh R, Forouhi M, Disfani HF, Kamandi M, Oskooi RK, Foogerdi M, Soltani M, Rahchamani M, Mohaddespour M, Dianatinasab M. Rahmani K, et al. Front Public Health. 2022 Aug 26;10:873596. doi: 10.3389/fpubh.2022.873596. eCollection 2022. Front Public Health. 2022. PMID: 36091533 Free PMC article. - Strategic silences, eroded trust: The impact of divergent COVID-19 vaccine sentiments on healthcare workers' relations with peers and patients.
Heyerdahl LW, Dielen S, Dodion H, Van Riet C, Nguyen T, Simas C, Boey L, Kattumana T, Vandaele N, Larson HJ, Grietens KP, Giles-Vernick T, Gryseels C. Heyerdahl LW, et al. Vaccine. 2023 Jan 23;41(4):883-891. doi: 10.1016/j.vaccine.2022.10.048. Epub 2022 Oct 27. Vaccine. 2023. PMID: 36319488 Free PMC article. - A Study to Assess Symptom Profile and Break Through Infections Among Health Care Workers Post Covid Vaccination at Tertiary Care Health Facility.
Malhotra V, Oberoi S, Khaira R, Balgir RS, Kaur B, Kaura S. Malhotra V, et al. Indian J Community Med. 2022 Jul-Sep;47(3):369-374. doi: 10.4103/ijcm.ijcm_1105_21. Epub 2022 Oct 10. Indian J Community Med. 2022. PMID: 36438513 Free PMC article. - Efficacy of COVID-19 vaccines: From clinical trials to real life.
Deplanque D, Launay O. Deplanque D, et al. Therapie. 2021 Jul-Aug;76(4):277-283. doi: 10.1016/j.therap.2021.05.004. Epub 2021 May 12. Therapie. 2021. PMID: 34049688 Free PMC article.
References
- WHO WHO Coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/
- Office for National Statistics Coronavirus (COVID-19) latest insights. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/...
- Medicines and Healthcare Regulatory Authority MHRA guidance on coronavirus (COVID-19) March 19, 2020. https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-c...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous