Synaptic Vesicle Protein 2A Expression in Glutamatergic Terminals Is Associated with the Response to Levetiracetam Treatment - PubMed (original) (raw)
Synaptic Vesicle Protein 2A Expression in Glutamatergic Terminals Is Associated with the Response to Levetiracetam Treatment
Itzel Jatziri Contreras-García et al. Brain Sci. 2021.
Abstract
Synaptic vesicle protein 2A (SV2A), the target of the antiepileptic drug levetiracetam (LEV), is expressed ubiquitously in all synaptic terminals. Its levels decrease in patients and animal models of epilepsy. Thus, changes in SV2A expression could be a critical factor in the response to LEV. Epilepsy is characterized by an imbalance between excitation and inhibition, hence SV2A levels in particular terminals could also influence the LEV response. SV2A expression was analyzed in the epileptic hippocampus of rats which responded or not to LEV, to clarify if changes in SV2A alone or together with glutamatergic or GABAergic markers may predict LEV resistance. Wistar rats were administered saline (control) or pilocarpine to induce epilepsy. These groups were subdivided into untreated or LEV-treated groups. All epileptic rats were video-monitored to assess their number of seizures. Epileptic rats with an important seizure reduction (>50%) were classified as responders. SV2A, vesicular γ-aminobutyric acid transporter and vesicular glutamate transporter (VGLUT) expression were assessed by immunostaining. SV2A expression was not modified during epilepsy. However, responders showed ≈55% SV2A-VGLUT co-expression in comparison with the non-responder group (≈40%). Thus, SV2A expression in glutamatergic terminals may be important for the response to LEV treatment.
Keywords: SV2A; VGAT; VGLUT; hippocampus; levetiracetam; pharmacoresistance; temporal lobe epilepsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
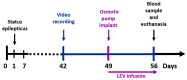
Figure 1
Experimental design. Status epilepticus (SE) was induced on day 1. Subsequently, spontaneous recurrent seizures were recorded before (days 42–48) and during (days 49–56) LEV treatment. A blood sample and the brain were obtained after humane euthanasia. LEV, levetiracetam.
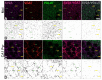
Figure 2
Example of immunofluorescent quantified puncta, using the Fiji plugin Synapse Counter, in high magnification images. (A,C) Confocal images of the molecular (Mol) and CA1 pyramidal (CA1 Pyr) layers with SV2A (purple), VGAT (red) or VGLUT (green) puncta, as well as SV2A-VGAT (magenta) or SV2A-VGLUT (gray) co-localized puncta. (B,D). Black and white images of puncta detected with Synapse Counter; each point corresponds to an immunofluorescent punctum. In each image, green arrows show SV2A-VGAT co-localized puncta, yellow arrows show SV2A-VGLUT co-localized puncta (scale bar = 10 µm).
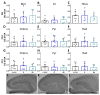
Figure 3
SV2A optical density (OD) after calibration and background subtraction. (A–C) Graphs of SV2A OD in the dentate gyrus (DG). (D–F) Graphs of SV2A OD in the CA3. (G–I) Graphs of SV2A OD in the CA1. The data are shown as mean ± SD (J–L). Representative microphotographs of SV2A OD in C+LEV (J), R (K), and NR (L) rats (scale bar = 500 µm).
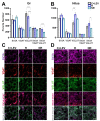
Figure 4
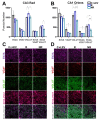
Quantification of SV2A, VGAT, VGLUT, SV2A-VGAT or SV2A-VGLUT puncta in the dentate gyrus granular layer (Gr) and hilus. (A,B) Graphs showing protein puncta (mean ± SD) in confocal images (50 × 50 µm) and their co-localization in C+LEV, R and NR animals. * p ≤ 0.05, *** p ≤ 0.001 (C,D) Confocal images of immunofluorescent SV2A (purple), VGAT (red) and VGLUT (green) puncta in the Gr and hilus, as well as SV2A-VGAT (magenta) or SV2A-VGLUT (gray) co-localized puncta in control plus levetiracetam (C+LEV), responder (R) and non-responder (NR) rats.
Figure 5
Quantification of SV2A, VGAT, VGLUT, SV2A-VGAT or SV2A-VGLUT puncta in the CA3 radiatum (CA3 Rad) and CA1 oriens. (A,B) Graphs showing protein puncta (mean ± SD) in confocal images (50 × 50 µm) and their co-localization in C+LEV, R and NR rats. * p ≤ 0.05, *** p ≤ 0.001 (C,D) Confocal images of immunofluorescent SV2A (purple), VGAT (red) and VGLUT (green) puncta in the Rad and oriens, as well as SV2A-VGAT (magenta) or SV2A-VGLUT (gray) co-localized puncta in control plus levetiracetam (C+LEV), responder (R) and non-responder (NR) rats.
Similar articles
- Differential expression of synaptic vesicle protein 2A after status epilepticus and during epilepsy in a lithium-pilocarpine model.
Contreras-García IJ, Pichardo-Macías LA, Santana-Gómez CE, Sánchez-Huerta K, Ramírez-Hernández R, Gómez-González B, Rocha L, Mendoza Torreblanca JG. Contreras-García IJ, et al. Epilepsy Behav. 2018 Nov;88:283-294. doi: 10.1016/j.yebeh.2018.08.023. Epub 2018 Oct 15. Epilepsy Behav. 2018. PMID: 30336420 - Levetiracetam resistance: Synaptic signatures & corresponding promoter SNPs in epileptic hippocampi.
Grimminger T, Pernhorst K, Surges R, Niehusmann P, Priebe L, von Lehe M, Hoffmann P, Cichon S, Schoch S, Becker AJ. Grimminger T, et al. Neurobiol Dis. 2013 Dec;60:115-25. doi: 10.1016/j.nbd.2013.08.015. Epub 2013 Sep 7. Neurobiol Dis. 2013. PMID: 24018139 - The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. Lynch BA, et al. Proc Natl Acad Sci U S A. 2004 Jun 29;101(26):9861-6. doi: 10.1073/pnas.0308208101. Epub 2004 Jun 21. Proc Natl Acad Sci U S A. 2004. PMID: 15210974 Free PMC article. - Therapeutic Role of Synaptic Vesicle Glycoprotein 2A (SV2A) in Modulating Epileptogenesis.
Ohno Y, Tokudome K. Ohno Y, et al. CNS Neurol Disord Drug Targets. 2017;16(4):463-471. doi: 10.2174/1871527316666170404115027. CNS Neurol Disord Drug Targets. 2017. PMID: 28393712 Review. - Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment.
Klitgaard H, Matagne A, Nicolas JM, Gillard M, Lamberty Y, De Ryck M, Kaminski RM, Leclercq K, Niespodziany I, Wolff C, Wood M, Hannestad J, Kervyn S, Kenda B. Klitgaard H, et al. Epilepsia. 2016 Apr;57(4):538-48. doi: 10.1111/epi.13340. Epub 2016 Feb 26. Epilepsia. 2016. PMID: 26920914 Review.
Cited by
- Levetiracetam Mechanisms of Action: From Molecules to Systems.
Contreras-García IJ, Cárdenas-Rodríguez N, Romo-Mancillas A, Bandala C, Zamudio SR, Gómez-Manzo S, Hernández-Ochoa B, Mendoza-Torreblanca JG, Pichardo-Macías LA. Contreras-García IJ, et al. Pharmaceuticals (Basel). 2022 Apr 13;15(4):475. doi: 10.3390/ph15040475. Pharmaceuticals (Basel). 2022. PMID: 35455472 Free PMC article. Review. - Changes in the Dentate Gyrus Gene Expression Profile Induced by Levetiracetam Treatment in Rats with Mesial Temporal Lobe Epilepsy.
Diaz-Villegas V, Pichardo-Macías LA, Juárez-Méndez S, Ignacio-Mejía I, Cárdenas-Rodríguez N, Vargas-Hernández MA, Mendoza-Torreblanca JG, Zamudio SR. Diaz-Villegas V, et al. Int J Mol Sci. 2024 Jan 30;25(3):1690. doi: 10.3390/ijms25031690. Int J Mol Sci. 2024. PMID: 38338984 Free PMC article. - Effect of Levetiracetam on Oxidant-Antioxidant Activity during Long-Term Temporal Lobe Epilepsy in Rats.
Ignacio-Mejía I, Contreras-García IJ, Pichardo-Macías LA, García-Cruz ME, Ramírez Mendiola BA, Bandala C, Medina-Campos ON, Pedraza-Chaverri J, Cárdenas-Rodríguez N, Mendoza-Torreblanca JG. Ignacio-Mejía I, et al. Int J Mol Sci. 2024 Aug 28;25(17):9313. doi: 10.3390/ijms25179313. Int J Mol Sci. 2024. PMID: 39273262 Free PMC article. - Synaptic Vesicle Glycoprotein 2A: Features and Functions.
Rossi R, Arjmand S, Bærentzen SL, Gjedde A, Landau AM. Rossi R, et al. Front Neurosci. 2022 Apr 28;16:864514. doi: 10.3389/fnins.2022.864514. eCollection 2022. Front Neurosci. 2022. PMID: 35573314 Free PMC article. Review. - Antiseizure medication in early nervous system development. Ion channels and synaptic proteins as principal targets.
Castro PA, Pinto-Borguero I, Yévenes GE, Moraga-Cid G, Fuentealba J. Castro PA, et al. Front Pharmacol. 2022 Oct 14;13:948412. doi: 10.3389/fphar.2022.948412. eCollection 2022. Front Pharmacol. 2022. PMID: 36313347 Free PMC article. Review.
References
- Pichardo L.A., Contreras I.J., Zamudio S.R., Mixcoha E., Mendoza J.G. Synaptic Vesicle Protein 2A as a novel pharma-cological target with broad potential for new antiepileptic drugs. In: Talevi A., Rocha L., editors. Antiepileptic Drug Discovery: Novel Approaches, Methods in Pharmacology and Toxicology. Springer; Berlin, Germany: 2016. pp. 53–65.
LinkOut - more resources
Full Text Sources