Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021 - PubMed (original) (raw)
. 2021 May 7;70(18):674-679.
doi: 10.15585/mmwr.mm7018e1.
Samantha M Olson, Wesley H Self, H Keipp Talbot, Christopher J Lindsell, Jay S Steingrub, Nathan I Shapiro, Adit A Ginde, David J Douin, Matthew E Prekker, Samuel M Brown, Ithan D Peltan, Michelle N Gong, Amira Mohamed, Akram Khan, Matthew C Exline, D Clark Files, Kevin W Gibbs, William B Stubblefield, Jonathan D Casey, Todd W Rice, Carlos G Grijalva, David N Hager, Arber Shehu, Nida Qadir, Steven Y Chang, Jennifer G Wilson, Manjusha Gaglani, Kempapura Murthy, Nicole Calhoun, Arnold S Monto, Emily T Martin, Anurag Malani, Richard K Zimmerman, Fernanda P Silveira, Donald B Middleton, Yuwei Zhu, Dayna Wyatt, Meagan Stephenson, Adrienne Baughman, Kelsey N Womack, Kimberly W Hart, Miwako Kobayashi, Jennifer R Verani, Manish M Patel; IVY Network; HAIVEN Investigators
Collaborators, Affiliations
- PMID: 33956782
- PMCID: PMC9368749
- DOI: 10.15585/mmwr.mm7018e1
Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021
Mark W Tenforde et al. MMWR Morb Mortal Wkly Rep. 2021.
Abstract
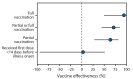
Adults aged ≥65 years are at increased risk for severe outcomes from COVID-19 and were identified as a priority group to receive the first COVID-19 vaccines approved for use under an Emergency Use Authorization (EUA) in the United States (1-3). In an evaluation at 24 hospitals in 14 states,* the effectiveness of partial or full vaccination† with Pfizer-BioNTech or Moderna vaccines against COVID-19-associated hospitalization was assessed among adults aged ≥65 years. Among 417 hospitalized adults aged ≥65 years (including 187 case-patients and 230 controls), the median age was 73 years, 48% were female, 73% were non-Hispanic White, 17% were non-Hispanic Black, 6% were Hispanic, and 4% lived in a long-term care facility. Adjusted vaccine effectiveness (VE) against COVID-19-associated hospitalization among adults aged ≥65 years was estimated to be 94% (95% confidence interval [CI] = 49%-99%) for full vaccination and 64% (95% CI = 28%-82%) for partial vaccination. These findings are consistent with efficacy determined from clinical trials in the subgroup of adults aged ≥65 years (4,5). This multisite U.S. evaluation under real-world conditions suggests that vaccination provided protection against COVID-19-associated hospitalization among adults aged ≥65 years. Vaccination is a critical tool for reducing severe COVID-19 in groups at high risk.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Christopher J. Lindsell reports grants from National Institutes of Health, U.S. Department of Defense, Marcus Foundation, Endpoint Health, Entegrion, bioMerieux, and Bioscape Digital, outside the submitted work. Jay S. Steingrub reports grants from National Institutes of Health, outside the submitted work. Akram Khan reports grants from United Therapeutics, Actelion Pharmaceuticals, Regeneron, and Reata Pharmaceuticals, outside the submitted work. Samuel M. Brown reports grants from National Institutes of Health, U.S. Department of Defense, Intermountain Research and Medical Foundation, and Janssen, and consulting fees paid to his employer from Faron and Sedana, all outside the submitted work. Ithan D. Peltan reports grants from National Institutes of Health and, outside the submitted work, grants from Asahi Kasei Pharma, Janssen Pharmaceuticals, and Regeneron. Adit A. Ginde reports grants from National Institutes of Health, U.S. Department of Defense and AbbVie, outside the submitted work. Carlos G. Grijalva reports consulting fees from Pfizer, Merck, and Sanofi-Pasteur, grants from Campbell Alliance/Syneos Health, National Institutes of Health, Food and Drug Administration, and Agency for Health Care Research and Quality, outside the submitted work. Michelle N. Gong reports grants from National Institutes of Health, Agency for Healthcare Research and Quality, and consulting fees from Regeneron, Philips Healthcare, all outside the submitted work. Steven Y. Chang reports consulting fees from PureTech Health and speaker fees from La Jolla Pharmaceuticals, both outside the submitted work. Jonathan D. Casey reports grants from National Institutes of Health, outside the submitted work. Todd W. Rice reports grants from National Institutes of Health and Endpoint Health, consulting work for Cumberland Pharmaceuticals, Inc, and Sanofi, Inc., outside the submitted work. Manjusha Gaglani reports grants from CDC-Abt Associates, outside the submitted work. Emily T. Martin reports personal fees from Pfizer and grants from Merck, outside the submitted work. Anurag Malani reports shareholder of Pfizer pharmaceuticals. Arnold S. Monto reports personal fees from Sanofi Pasteur and Seqirus, outside the submitted work. Fernanda P. Silveira reports grants from Shire, Qiagen, Ansun, and Novartis, outside the submitted work. Richard K. Zimmerman reports grants from Sanofi Pasteur, outside the submitted work. Donald B. Middleton reports grants and personal fees from Pfizer and personal fees from Seqirus, Sanofi Pasteur, and GlaxoSmithKline, outside the submitted work. No other potential conflicts of interest were disclosed.
Figures
FIGURE
Adjusted vaccine effectiveness (with 95% confidence intervals) against COVID-19 among hospitalized adults aged ≥65 years, by vaccination status — 24 medical centers in 14 states, January–March 2021 Abbreviations: HAIVEN = Hospitalized Adult Influenza Vaccine Effectiveness Network; IVY = Influenza and Other Viruses in the Acutely Ill. * Vaccine effectiveness estimates were adjusted for U.S. Census region, calendar month, continuous age in years, sex, race and ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic other or unknown, or Hispanic of any race), and one or more versus zero self-reported previous hospitalizations in the past year. † Clinical criteria for hospitalized COVID-19–like illness varied by hospital network. IVY Network criteria for COVID-19–like illness included presence of fever, feverishness, cough, sore throat, myalgias, shortness of breath, chest pain, loss of taste, loss of smell, respiratory congestion, increased sputum production, new oxygen saturation <94% on room air, new invasive or noninvasive ventilation, or new pulmonary findings on chest imaging consistent with pneumonia in the IVY Network; criteria included fever without a known non–COVID-19 cause, new or worsening cough, a change in sputum production, or new or worsening shortness of breath in the HAIVEN network. § SARS-CoV-2 vaccination status included the following four categories: 1) unvaccinated, defined as no receipt of any SARS CoV-2 vaccine; 2) first vaccine dose <14 days before illness onset, defined as a single dose of vaccine within 14 days prior to onset of COVID-19–like illness; 3) partially vaccinated, defined as receipt of 1 dose of a 2-dose vaccine series (Pfizer-BioNTech or Moderna) ≥14 days before illness onset or 2 doses with the second dose received <14 days before illness onset); 4) fully vaccinated, defined as receipt of both doses of a 2-dose vaccine series ≥14 days before illness onset. ¶ Patients were enrolled from 24 medical centers in 14 states (University of California Los Angeles and Stanford University [California], UCHealth University of Colorado Hospital [Colorado], Johns Hopkins Hospital [Maryland], Beth Israel Deaconess Medical Center and Baystate Medical Center [Massachusetts], University of Michigan, Henry Ford, and St. Joseph [Michigan], Hennepin County Medical Center [Minnesota], Montefiore Healthcare Center [New York], Wake Forest University [North Carolina], Ohio State University [Ohio], Oregon Health & Science University [Oregon], University of Pittsburgh Medical Center, Shadyside, Mercy, Passavant, St. Margaret, and Presbyterian Hospitals [Pennsylvania], Vanderbilt University Medical Center [Tennessee], Baylor Scott & White Medical Center, Temple, Round Rock, Hillcrest/Waco [Texas], and Intermountain Health [Utah]).
Similar articles
- Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021.
Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Lindsell CJ, Hart KW, Zhu Y, Mills L, Lester SN, Stumpf MM, Naioti EA, Kobayashi M, Verani JR, Thornburg NJ, Patel MM; IVY Network. Self WH, et al. MMWR Morb Mortal Wkly Rep. 2021 Sep 24;70(38):1337-1343. doi: 10.15585/mmwr.mm7038e1. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34555004 Free PMC article. - Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021.
Tenforde MW, Self WH, Naioti EA, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, Zepeski A, Gaglani M, McNeal T, Ghamande S, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Lindsell CJ, Hart KW, Zhu Y, Stephenson M, Schrag SJ, Kobayashi M, Verani JR, Patel MM; IVY Network Investigators; IVY Network. Tenforde MW, et al. MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1156-1162. doi: 10.15585/mmwr.mm7034e2. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34437524 Free PMC article. - Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years - COVID-NET, 13 States, February-April 2021.
Moline HL, Whitaker M, Deng L, Rhodes JC, Milucky J, Pham H, Patel K, Anglin O, Reingold A, Chai SJ, Alden NB, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Farley MM, Ryan PA, Kim S, Nunez VT, Como-Sabetti K, Lynfield R, Sosin DM, McMullen C, Muse A, Barney G, Bennett NM, Bushey S, Shiltz J, Sutton M, Abdullah N, Talbot HK, Schaffner W, Chatelain R, Ortega J, Murthy BP, Zell E, Schrag SJ, Taylor C, Shang N, Verani JR, Havers FP. Moline HL, et al. MMWR Morb Mortal Wkly Rep. 2021 Aug 13;70(32):1088-1093. doi: 10.15585/mmwr.mm7032e3. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34383730 Free PMC article. - COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.
Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. Meo SA, et al. Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review. - Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review.
Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Pillay J, et al. BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
Cited by
- COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infections, COVID-19 related hospitalizations and deaths, among individuals aged ≥65 years in Portugal: A cohort study based on data-linkage of national registries February-September 2021.
Machado A, Kislaya I, Rodrigues AP, Sequeira D, Lima J, Cruz C, Leite PP, Matias Dias C, Nunes B. Machado A, et al. PLoS One. 2022 Sep 13;17(9):e0274008. doi: 10.1371/journal.pone.0274008. eCollection 2022. PLoS One. 2022. PMID: 36099273 Free PMC article. - Outcomes of COVID-19 in heart failure, LVAD, and heart transplant patients in an advanced heart failure practice.
George S, Cunningham LC, Nelson DP, Horstmanshof DA, Long JW, El Banayosy AM. George S, et al. Am Heart J Plus. 2022 Dec;24:100223. doi: 10.1016/j.ahjo.2022.100223. Epub 2022 Nov 3. Am Heart J Plus. 2022. PMID: 36345551 Free PMC article. - Pfizer-BioNTech (BNT162b2) Vaccine Effectiveness against Symptomatic Laboratory-Confirmed COVID-19 Infection among Outpatients in Sentinel Sites, Lebanon, July-December 2021.
Chaito L, Stefanoff P, Baruch J, Farah Z, Albuaini M, Ghosn N. Chaito L, et al. Vaccines (Basel). 2024 Aug 23;12(9):954. doi: 10.3390/vaccines12090954. Vaccines (Basel). 2024. PMID: 39339986 Free PMC article. - The long-term effectiveness of coronavirus disease 2019 (COVID-19) vaccines: A systematic literature review and meta-analysis.
Marra AR, Kobayashi T, Suzuki H, Alsuhaibani M, Schweizer ML, Diekema DJ, Tofaneto BM, Bariani LM, Auler MA, Salinas JL, Edmond MB, Pinho JRR, Rizzo LV. Marra AR, et al. Antimicrob Steward Healthc Epidemiol. 2022 Feb 14;2(1):e22. doi: 10.1017/ash.2021.261. eCollection 2022. Antimicrob Steward Healthc Epidemiol. 2022. PMID: 36310810 Free PMC article. Review. - Estimating COVID-19 Vaccination and Booster Effectiveness Using Electronic Health Records From an Academic Medical Center in Michigan.
Roberts EK, Gu T, Wagner AL, Mukherjee B, Fritsche LG. Roberts EK, et al. AJPM Focus. 2022 Sep;1(1):100015. doi: 10.1016/j.focus.2022.100015. Epub 2022 Jul 26. AJPM Focus. 2022. PMID: 36942016 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- U01 IP000972/IP/NCIRD CDC HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U01 IP000969/IP/NCIRD CDC HHS/United States
- U01 IP000974/IP/NCIRD CDC HHS/United States
- K23 GM129661/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical