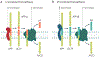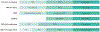Alzheimer disease - PubMed (original) (raw)
Review
Alzheimer disease
David S Knopman et al. Nat Rev Dis Primers. 2021.
Abstract
Alzheimer disease (AD) is biologically defined by the presence of β-amyloid-containing plaques and tau-containing neurofibrillary tangles. AD is a genetic and sporadic neurodegenerative disease that causes an amnestic cognitive impairment in its prototypical presentation and non-amnestic cognitive impairment in its less common variants. AD is a common cause of cognitive impairment acquired in midlife and late-life but its clinical impact is modified by other neurodegenerative and cerebrovascular conditions. This Primer conceives of AD biology as the brain disorder that results from a complex interplay of loss of synaptic homeostasis and dysfunction in the highly interrelated endosomal/lysosomal clearance pathways in which the precursors, aggregated species and post-translationally modified products of Aβ and tau play important roles. Therapeutic endeavours are still struggling to find targets within this framework that substantially change the clinical course in persons with AD.
Figures
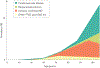
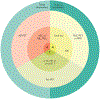
Fig. 1 ∣. Neuropathological diagnoses that cause cognitive impairment across the age spectrum.
Alzheimer disease (AD) may occur without other co-pathologies in those <70 years of age but other pathologies are common in older individuals with AD pathology. DLB, dementia with Lewy bodies; FTLD, frontotemporal lobar degeneration. Adapted from REF., Springer Nature Limited.
Fig. 2 ∣. APP cleavage pathways.
a ∣ In the non-β-amyloid (Aβ) pathway, successive cleavage by α-secretase leads to the formation of APPsα and αCTF, which in turn is cleaved by γ-secretase to yield the extracellular peptide p3 and the intracellular fragment AICD. APPsα may modulate synaptic transmission through a GABA receptor. b ∣ Aβ is formed in the ‘amyloidogenic’ pathway by the successive cleavage of amyloid precursor protein (APP) by β-secretase into APPsβ and βCTF, the latter being then subjected to γ-secretase, producing Aβ and AICD. βCTF plays a key role in early endosomal abnormalities in AD. Note that the production of Aβ is necessarily in equal amounts to AICD and APPsβ and in inverse amounts to p3 and APPsα. Adapted with permission from REF., Elsevier.
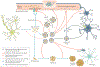
Fig. 3 ∣. ApoE and Aβ interaction.
In one pathway, monomeric β-amyloid (Aβ) aggregates into oligomers, then into fibrils and finally becomes part of amyloid plagues. ABCA1 participates in the lipidation of ApoE, which has a strong effect on Aβ fibrillization. ApoE complexes with Aβ monomers and, by interacting with the low-density lipoprotein receptor (LDLR) and LDL receptor-related protein 1 (LRP1), transports Aβ into the perivascular spaces around arterioles and venules for removal by the glymphatic pathway. Heparan sulfate proteoglycan (HSPG) also interacts with LRP1 and Aβand influences its oligomerization. Within the perivascular space, Aβ can aggregate, which leads to cerebral amyloid angiopathy (CAA), which in turn can damage the vascular wall, leading to micro haemorrhages. Through the triggering receptor expressed on myeloid cells 2 (TREM2), lipoproteins, including ApoE, influence the phagocytic properties of microglia and might influence plague removal. Adapted with permission from REF., Elsevier.
Fig. 4 ∣. Consequences of the endosomal–lysosomal network and autophagy dysfunction in AD.
The endosomal–lysosomal pathway, consisting of early and late endosomes/multivesicular bodies and lysosomes, serves diverse functions in neurons relevant to Alzheimer disease (AD). In AD, the earliest changes seen in the brain are swelling of neuronal Rab5-endosomes, reflecting the hyper-activation of Rab5 (Rab5-GTP) by amyloid precursor protein (APP)-βCTF or neuronal overexpression of Rab5. Increased Rab5 activation leads to endosome enlargement and increased endocytosis, which have several cellular consequences relevant for AD. For example, increased endocytosis of AMPA receptors (AMPAR) leads to defects in synaptic plasticity and dendritic spine shrinkage and loss. In addition, abnormal growth factor/receptor-mediated signalling results in the downregulation of AKT-mediated prosurvival signalling and increased GSK-3β-mediated tau hyperphosphorylation (p-tau). Corresponding endosomal–lysosomal activities in astrocytes and microglia coordinate bi-directional trafficking of cargo into and out of cells to maintain them and support the clearance of extracellular material in partnership with autophagy. Autophagy encompasses several mechanisms of constituent delivery to lysosomes, including initial entry of the substrate into late endosomes/multivesicular bodies (microautophagy), chaperone-mediated delivery directly to lysosomes, or macroautophagy, the major route depicted here. The increased induction of autophagy, a cellular stress response, becomes counter-productive as the functioning of autolysosomes and lysosomes is progressively corrupted due to multiple genetic and environmental factors. The result is a substantial build-up of autophagic vacuoles, mainly poorly acidified autolysosomes incompetent in clearing Aβ and βCTF, causing a unique pattern of perikaryal membrane blebbing, trafficking deficits producing autophagic vacuole-filled swellings along axons (dystrophic neurites), and accelerated peri-nuclear Aβ aggregation preceding advanced neuronal degeneration and disintegration, initiating senile plaque formation. An ensuing inflammatory response involving the recruitment of phagocytic microglia to compromised neurons/neurites and a release of damaging cytokines as the extracellular debris is being cleared increases bystander neurotoxicity, affecting neighbouring neurons and senile plaque expansion. LMP, lysosomal membrane permeabilization; LTD, long-term depression; LTP, long-term potentiation; ASC, Aβoptosis-associated speck-like protein.
Fig. 5 ∣. Terminologies for characterizing cognitive impairment.
Several different terminology schemes can be used to classify the severity of cognitive impairment from cognitively normal (CN) to dementia. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) uses the terms Mild Neurocognitive Disorder (NCD) and Major NCD to describe symptomatic states. The National Institute of Ageing – Alzheimer’s Association (NIA-AA) Alzheimer disease (AD) framework uses clinical syndromes and defines a six-stage scheme for individuals who have abnormal β-amyloid biomarkers (ST1–ST6). In the NIA-AA scheme, mild cognitive impairment (MCI) encompasses both stages 2 and 3. The NIA-AA continuum,, has evolved slightly at the boundary between cognitively unimpaired (CU) and MCI. The International Work Group (IWG) labels were retrieved from REF.. The terms CN and CU are equivalent, but the latter is preferred because CU better reflects its definition as the absence of cognitive impairment.
Fig. 6 ∣. Conceptualizing the A-T-N scheme.
Cerebrospinal fluid and PET imaging biomarkers can be grouped into those that proxy amyloid-β (Aβ; “A”), abnormal tau protein (“T”) or neurodegeneration (“N”). The A biomarkers derived from PET and cerebrospinal fluid (CSF) have an inverse relationship such that higher Aβ-PET corresponds to lower levels of CSF Aβ. The “T” biomarkers derived from PET and CSF are both abnormal at higher values. Quantitatively, tau-PET provides both a measure of regional tau abundance and distribution, whereas CSF p-tau181 or p-tau217 offer only a metric of normal or abnormal tau levels. The “N” markers, by virtue of their lack of specificity to Alzheimer disease (AD) and their heterogeneous underlying biologies, are generally not well correlated with one another. Biomarkers for AD can indicate whether a person is in the AD spectrum (state biomarkers) that include Aβ abnormalities and elevated tau biomarkers. Biomarkers can also be used to indicate the severity of the AD process (stage biomarkers) that include tau-PET and the neurodegeneration biomarkers. NfL, neurofilament light chain.
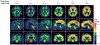
Fig. 7 ∣. Aβ-PET scans closely approximate neuropathology.
Findings from antemortem 11C Pittsburgh Compound B (PiB) scans showing different standardized uptake value ratios (SUVR) of a global region of interest on the last scan prior to death are similar to findings from post-mortem-derived amyloid-β (Aβ) burden as rated on the “Thal phase”. The Thal Aβ staging system is a sequence of five levels of Aβ accumulation that reflect the expanding territory occupied by Aβ plaques. The relevant PiB signal is on the cortical surface, whereas binding in the subcortical white matter and brainstem represents the non-specific binding of the tracer. Adapted with permission from REF., Oxford University Press.
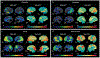
Fig. 8 ∣. Tau-PET and FDG-PET patterns in different clinical syndromes in persons with high β-amyloid-PET.
a ∣ Typical amnestic-predominant Alzheimer disease with temporoparietal hypometabolism and tauopathy. b ∣ Language syndrome (also known as logopenic variant primary progressive aphasia) with a highly asymmetric pattern in which hypometabolism and tauopathy are highly left hemisphere predominant. c ∣ Visual syndrome (also known as posterior cortical atrophy) with a pattern of hypometabolism and tauopathy that is posterior temporal, parietal and occipital lobar in distribution. d ∣ Dysexecutive syndrome with temporal, parietal and prominent frontal hypometabolism and tauopathy. Red colour on FDG-PET indicates greater hypometabolism, whereas red colour on tau-PET indicates a higher intensity of tracer retention. LL, left lateral; LM, left medial; RL, right lateral; RM, right medial.
Similar articles
- Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis.
Bloom GS. Bloom GS. JAMA Neurol. 2014 Apr;71(4):505-8. doi: 10.1001/jamaneurol.2013.5847. JAMA Neurol. 2014. PMID: 24493463 Review. - Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.
Torres AK, Jara C, Park-Kang HS, Polanco CM, Tapia D, Alarcón F, de la Peña A, Llanquinao J, Vargas-Mardones G, Indo JA, Inestrosa NC, Tapia-Rojas C. Torres AK, et al. J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review. - Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD.
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA. Tramutola A, et al. J Neurochem. 2015 Jun;133(5):739-49. doi: 10.1111/jnc.13037. Epub 2015 Feb 26. J Neurochem. 2015. PMID: 25645581 - Recent update on the heterogeneity of the Alzheimer's disease spectrum.
Jellinger KA. Jellinger KA. J Neural Transm (Vienna). 2022 Jan;129(1):1-24. doi: 10.1007/s00702-021-02449-2. Epub 2021 Dec 17. J Neural Transm (Vienna). 2022. PMID: 34919190 Review. - Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia.
Ferrer I. Ferrer I. Prog Neurobiol. 2012 Apr;97(1):38-51. doi: 10.1016/j.pneurobio.2012.03.005. Epub 2012 Mar 21. Prog Neurobiol. 2012. PMID: 22459297 Review.
Cited by
- Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain.
Taing K, Chen L, Weng HR. Taing K, et al. Neural Regen Res. 2023 Apr;18(4):763-768. doi: 10.4103/1673-5374.354514. Neural Regen Res. 2023. PMID: 36204834 Free PMC article. Review. - Can Activation of Acetylcholinesterase by β-Amyloid Peptide Decrease the Effectiveness of Cholinesterase Inhibitors?
Zueva IV, Vasilieva EA, Gaynanova GA, Moiseenko AV, Burtseva AD, Boyko KM, Zakharova LY, Petrov KA. Zueva IV, et al. Int J Mol Sci. 2023 Nov 16;24(22):16395. doi: 10.3390/ijms242216395. Int J Mol Sci. 2023. PMID: 38003588 Free PMC article. - Astrocytes Excessively Engulf Synapses in a Mouse Model of Alzheimer's Disease.
Li L, Lu S, Zhu J, Yu X, Hou S, Huang Y, Niu X, Du X, Liu R. Li L, et al. Int J Mol Sci. 2024 Jan 18;25(2):1160. doi: 10.3390/ijms25021160. Int J Mol Sci. 2024. PMID: 38256233 Free PMC article. - Enabling uncertainty estimation in neural networks through weight perturbation for improved Alzheimer's disease classification.
Ferrante M, Boccato T, Toschi N. Ferrante M, et al. Front Neuroinform. 2024 Feb 6;18:1346723. doi: 10.3389/fninf.2024.1346723. eCollection 2024. Front Neuroinform. 2024. PMID: 38380126 Free PMC article. - Simvastatin, Its Antimicrobial Activity and Its Prevention of Alzheimer's Disease.
Dhakal S, Macreadie IG. Dhakal S, et al. Microorganisms. 2024 Jun 1;12(6):1133. doi: 10.3390/microorganisms12061133. Microorganisms. 2024. PMID: 38930515 Free PMC article. Review.
References
- Petersen RC Mild cognitive impairment as a diagnostic entity. J. Intern. Med 256, 183–194 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG062376/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- P01 AG017617/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical