Adrenaline May Contribute to Prothrombotic Condition via Augmentation of Platelet Procoagulant Response, Enhancement of Fibrin Formation, and Attenuation of Fibrinolysis - PubMed (original) (raw)
Adrenaline May Contribute to Prothrombotic Condition via Augmentation of Platelet Procoagulant Response, Enhancement of Fibrin Formation, and Attenuation of Fibrinolysis
Agata Golaszewska et al. Front Physiol. 2021.
Abstract
Background: Adrenaline is believed to play a role in thrombosis and hemostasis. The complex effect of its clinically relevant concentrations on thrombus formation, coagulation and fibrinolysis in human blood has never been specifically studied. Methods: Confocal microscopy was used to study thrombus formation under flow, exposure of phosphatidylserine (PS) in adhered platelets, to evaluate clots density, and to measure kinetics of fibrin formation and external fibrinolysis under flow. Flow cytometry was utilized to assess PS exposure in non-adhered platelets. Kinetics of clot formation and internal fibrinolysis was evaluated by thromboelastometry. Platelet aggregation was measured by optical aggremometry. Kinetics of clot retraction was assessed by using digital camera. Results: We found that adrenaline (1-10 nM) is able to enhance platelet activation evoked by subthreshold collagen (150 ng/ml), resulting in augmentation of platelet aggregation, thrombus formation under arterial flow conditions, platelet PS exposure, and formation of platelet-fibrin clots. The development of platelet procoagulant response evoked by adrenaline + low collagen was associated with the formation of denser platelet-fibrin clots and the decrease in rate of fibrinolysis despite whether lysis was initiated inside (internal fibrinolysis) or outside the clot (external fibrinolysis). The above phenomena were abolished by the α2-adrenergic receptor antagonist, rauwolscine. Adrenaline-collagen synergism, expressed as PS exposure, was significantly reduced by cyclooxygenase inhibitor (acetylsalicic acid), GPIIb/IIIa receptor blocker (tirofiban), and P2Y12 receptor antagonist (PSB 0739). Conclusion: Clinically relevant concentrations of adrenaline may significantly augment responses of human platelets in the presence of subthreshold concentrations of collagen, which should be considered during therapies involving adrenaline infusion. Routinely used antiplatelet drugs may reduce the prothrombotic state evoked by adrenaline-collagen synergism.
Keywords: adrenaline; fibrinolysis; phosphatidylserine exposure; platelet; procoagulant activities; thrombus formation.
Copyright © 2021 Golaszewska, Misztal, Marcinczyk, Chabielska and Rusak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
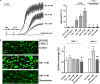
FIGURE 1
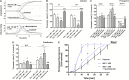
Effect of nanomolar adrenaline concentrations on platelet aggregation and thrombus formation under flow (A). Samples of PRP were incubated without or with rauwolscine (10 μM, for 10 min) and supplemented with adrenaline (1–10 nM), collagen (150 ng/ml) or combination of both agonists (in that case adrenaline was added first, 1 min before collagen). Representative aggregation curves from one (out of 6) experiment (one experiment is considered as aggregation measurements—at least in three repetitions—using PRP obtained from one donor) and aggregation extent are presented in (A). Aggregation obtained in the presence of 5 μg/ml of collagen was considered as maximal aggregation. Adrenaline concentrations below 10 nM also did not trigger platelet aggregation (not shown). Data are means ± S.D. from n = 6 experiments. *p < 0.05 vs. “Coll 150 ng/ml.” #p < 0.05 vs. sample containing collagen (150 ng/ml) and appropriate concentration of adrenaline. (B) PPACK-anticoagulated whole blood samples (supplemented with DiO to visualize platelets) were incubated without any addition (control) or with adrenaline (for 2 min) or rauwolscine (10 μM, for 10 min) + adrenaline (for 2 min). Next, samples were perfused over collagen-coated surfaces at shear rate 1,000 or 1,600 s– 1 to form thrombi. Surface coverage area was calculated from end-stage confocal pictures. Data are means ± S.D. from n = 9 experiments. **p < 0.01 vs. control; #p < 0.05, ##p < 0.01 vs. adrenaline 1 nM. Presented pictures are representative for experiments conducted at shear rate 1,000 s– 1.
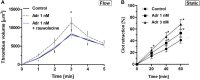
FIGURE 2
Effect of nanomolar adrenaline concentrations on clot retraction. (A) To quantify thrombus volume, whole blood, preincubated with adrenaline, with rauwolscine + adrenaline or without any additions (control) was perfused over collagen at shear rate of 1,000 s– 1 and z-stacks were captured in real-time using a confocal microscope. Changes in thrombus volume was quantified using SlideBook 6 software. Data are means ± S.D. from n = 9 experiments. *p < 0.05 vs. control. (B) Samples of washed platelets were preincubated without (control) or with adrenaline (for 2 min) followed by a transfer to Tyrode’s buffer containing CaCl2 (2.5 mM) and ADP (2 μM, to stimulate platelet contractility) and batroxobin (5 BU/ml, to evoke coagulation without additional platelet activation). Decrease in clots volume was recorded by digital camera in 10 min intervals up to 60 min and expressed as clot retraction%. No retraction was noticed in the absence of ADP and adrenaline in the above experimental system (not shown). Data are means ± S.D. from n = 3 experiments. *p < 0.05 vs. control.
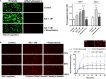
FIGURE 3
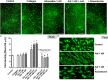
Effect of adrenaline on platelet PS exposure under flow in the absence or presence of thrombin. (A) PPACK-anticoagulated whole blood samples (supplemented with DiO to visualize platelets) were incubated without any addition (control) or with adrenaline (for 2 min) or rauwolscine (10 μM, for 10 min) + adrenaline (for 2 min). Next, samples were perfused over collagen-coated surfaces at shear rate 1,000 or 1,600 s– 1 to form thrombi. Thrombi were post-stained with AF647 annexin V to visualize platelet membrane-exposed phosphatidylserine (PS). Ratio of annexin-related fluorescence to DiO fluorescence, i.e., procoagulant index, was calculated from end-stage confocal pictures. Data are means ± S.D. from 9 experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control; ##p < 0.01 vs. adrenaline 1 nM. Presented pictures are representative for experiments conducted at shear rate 1,600 s– 1. (B) Whole blood samples (without any direct thrombin inhibitor) were incubated without any addition (control) or with adrenaline (for 2 min) or rauwolscine (10 μM, for 10 min) + adrenaline (for 2 min). Next, samples were perfused over collagen-coated surfaces at shear rate 1,000 s– 1 to form thrombi. No significant differences in surface area coverage by platelets between conditions with and without coagulation were observed. Afterward, thrombi were washed with AF647 annexin V and progress of its accumulation on the surface of PS-exposed platelets was recorded using confocal microscope. Representative pictures reflecting progress of annexin V binding (associated with 10, 50, and 100% of total annexin V fluorescence in appropriate sample) are presented (n = 4). Typical picture of balloon platelets formed in the absence (Ctrl) or in presence of 1 nM adrenaline (Adr) is shown as the insert in chart. For kinetics measurements, raw fluorescence intensities from time-series records (measurements in 1 min intervals) were quantified after regions of interest were chosen and images corrected for background noise. For each record, relative fluorescence (F/_F_0) is reported, where _F_0 designates the background-subtracted fluorescence level before platelet activation. Data are expressed as means ± S.D. from 4 experiments. *p < 0.05 vs. control; #p < 0.05 vs. adrenaline 1 nM. In panel B, *p < 0.05 vs. control (with coagulation). In all panels white bar = 10 μm.
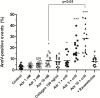
FIGURE 4
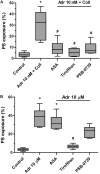
Effect of adrenaline on PS exposure in platelet suspension. PRP samples were incubated without any additions (control), with or without rauwolscine (10 μM, for 10 min) followed by the addition of adrenaline (1–10 nM), collagen (150 ng/ml) or combination of both agonists with initial stirring (30 s). After incubation (15 min at room temperature), samples were supplemented with appropriate fluorescent probes, and after final dilution analyzed toward PS exposure by flow cytometry (n = 16). *p < 0.05, ***p < 0.001 vs. control; #p < 0.001 vs. adrenaline 10 nM + coll.
FIGURE 5
Reduction of adrenaline and collagen-evoked platelet PS exposure by cyclooxygenase inhibitor, GPIIb/IIIa blocker, and P2Y12 receptor antagonist. Samples of PRP were incubated for 10 min with cyclooxygenase inhibitor (ASA, 200 μM), GPIIb/IIIa blocker (tirofiban, 500 nM), and P2Y12 receptor antagonist (PSB-0739, 100 μM). Next, samples were supplemented with adrenaline (10 nM) and collagen (150 ng/ml, A) or with adrenaline alone (10 μM, B). Samples were incubated at room temperature for 30 min. Attention was taken to do not allow platelets to form aggregates. Control samples were without any addition. After incubation, samples were supplemented with appropriate fluorescent probes, and after final dilution analyzed toward PS exposure by flow cytometry. Presented results are medians with interquartile range from 6 independent experiments. *p < 0.05 vs. control in both panels; #p < 0.05 vs. Adr 10 nM + coll in (A) and p < 0.05 vs. Adr 10 μM in (B).
FIGURE 6
Effect of adrenaline on the kinetics of platelet-fibrin clot formation. Whole blood samples were incubated with or without rauwolscine (10 μM, for 10 min) followed by the addition of adrenaline (for 2 min) and collagen (150 ng/ml) with initial stirring (30 s). After 10 min of incubation, at room temperature, samples were analyzed toward kinetics of clot formation using rotational thromboelastometry. Clotting was initiated by recalcification (12 mM CaCl2 final conc.). Control samples were without any addition. Representative coagulation profiles and records of clot formation rate (CFR) for 6 experiments are presented in (A). (B–D) Parameters associated with clotting initiation (CT, clotting time; CFT, clot formation time), propagation (alpha angle; MaxV, maximal velocity of clot formation; MaxV-t, time to reach maximal velocity of clotting), and stabilization (MCF, maximum clot firmness; G, shear modulus strength) were measured. Data are means ± S.D. from 6 experiments. Parameters value range in control was: CT: 215–667 s; CFT: 79–176 s; Alpha: 64–74°; MaxV: 12–18 mm*100/s; MaxV-t: 351–855 s; MCF: 65–75 mm; G: 936–1,492. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. adrenaline 10 nM + coll. (E) Whole blood samples (supplemented with AF488-fibrinogen to visualize fibrin formation) were incubated without any addition (control) or with adrenaline (for 2 min) or rauwolscine (10 μM, for 10 min) + adrenaline (for 2 min). Kinetics of fibrin formation within thrombi, formed under flow (1,000 s– 1) on collagen-coated surfaces, was recorded in confocal microscope. The fibrin(ogen)-associated fluorescence was recorded and expressed as% of maximal fluorescence obtained (considered as 100%) vs. time. Data are means ± S.D. from 4 experiments. *p < 0.05 vs. control; #p < 0.05 vs. adrenaline 1 nM.
FIGURE 7
Effect of adrenaline on the architecture of platelet-fibrin clots under static and flow conditions. (A) PRP samples were incubated without any additions (control) or with or without rauwolscine (10 μM, for 10 min) followed by the addition of adrenaline (1–10 nM) + collagen (150 ng/ml). Afterward, samples were supplemented with AF488-fibrinogen (to visualize fibrin formation). Clotting was triggered by recalcification (18 mM CaCl2, final conc.) and resulted platelet-fibrin clots were analyzed under confocal microscope toward fibrin density. Structures representative for 7 experiments are presented in (A). Bars are means ± S.D. from 7 experiments. *p < 0.01 vs. control; #p < 0.01 vs. appropriate samples containing adrenaline and collagen. (B) Whole blood samples (supplemented with AF488-fibrinogen) were incubated without any addition (control) or with adrenaline (1 nM, for 2 min) or rauwolscine (10 μM, for 10 min) + adrenaline (for 2 min). Architecture of fibrin mesh within thrombi, formed under flow (1,000 s– 1) on collagen, was recorded in confocal microscope. Pictures are representative for 4 experiments. White bar is 30 μm in (A) and 10 μm in (B).
FIGURE 8
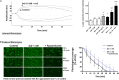
Effect of adrenaline on the kinetics of fibrinolysis. (A) Whole blood samples were supplemented with adrenaline (1–10 nM), collagen (150 ng/ml), or their combinations. After 10 min of incubation samples were mixed with tissue factor (100 ng/ml) and tPA (120 ng/ml) and kinetics of internal fibrinolysis [expressed as lysis onset time (LOT)] was evaluated by thromboelastometry. Presented records are representative for 6 experiments. LOT values in controls were in a range of 3,338–4,158 s. Bars are means ± S.D. from 6 experiments. *p < 0.05, **p < 0.01 vs. control; #p < 0.05 vs. collagen. (B) Whole blood samples were incubated without any additions (control) or with or without rauwolscine (10 μM, for 10 min) followed by the addition of adrenaline (1 nM, for 2 min). After addition of AF488-fibrinogen samples were perfused over collagen-coated surfaces at shear rate 1,000 s– 1. After formation of fibrin, clots were washed with a buffer containing tPA (40 nM) and sets of confocal pictures were collected until fibrin-related fluorescent signal decreased to the background level (reflecting progress of external fibrinolysis). Representative pictures of fluorescent fibrin at time points associated with the 10, 50, and 100% of lysis (related to control samples) and kinetics of fibrinolysis are presented. “t = 0” means fibrin(ogen)-related fluorescence at start point of fibrinolysis (considered as 100%). Values range in control samples were: to obtain 50% of lysis–407–1,103 s, to 100% of lysis–1,575–2,508 s. *p < 0.05 vs. control; #p < 0.05 vs. adrenaline. White bar = 10 μm.
Similar articles
- The myeloperoxidase product, hypochlorous acid, reduces thrombus formation under flow and attenuates clot retraction and fibrinolysis in human blood.
Misztal T, Golaszewska A, Tomasiak-Lozowska MM, Iwanicka M, Marcinczyk N, Leszczynska A, Chabielska E, Rusak T. Misztal T, et al. Free Radic Biol Med. 2019 Sep;141:426-437. doi: 10.1016/j.freeradbiomed.2019.07.003. Epub 2019 Jul 4. Free Radic Biol Med. 2019. PMID: 31279970 - Peroxynitrite may affect fibrinolysis via the reduction of platelet-related fibrinolysis resistance and alteration of clot structure.
Misztal T, Rusak T, Brańska-Januszewska J, Ostrowska H, Tomasiak M. Misztal T, et al. Free Radic Biol Med. 2015 Dec;89:533-47. doi: 10.1016/j.freeradbiomed.2015.09.006. Epub 2015 Oct 8. Free Radic Biol Med. 2015. PMID: 26454084 - HAuCl4, Putative General Aquaporins Blocker, Reduces Platelet Spreading, Filopodia Formation, Procoagulant Response, and Thrombus Formation Under Flow.
Misztal T, Golaszewska A, Branska-Januszewska J, Marcinczyk N, Chabielska E, Tomasiak M, Rusak T. Misztal T, et al. Front Physiol. 2020 Aug 21;11:1025. doi: 10.3389/fphys.2020.01025. eCollection 2020. Front Physiol. 2020. PMID: 32973556 Free PMC article. - Insights into platelet-based control of coagulation.
de Witt SM, Verdoold R, Cosemans JM, Heemskerk JW. de Witt SM, et al. Thromb Res. 2014 May;133 Suppl 2:S139-48. doi: 10.1016/S0049-3848(14)50024-2. Thromb Res. 2014. PMID: 24862135 Review. - Fibrinolysis in Platelet Thrombi.
Kanji R, Gue YX, Memtsas V, Gorog DA. Kanji R, et al. Int J Mol Sci. 2021 May 12;22(10):5135. doi: 10.3390/ijms22105135. Int J Mol Sci. 2021. PMID: 34066261 Free PMC article. Review.
Cited by
- Identification of the Antithrombotic Mechanism of Leonurine in Adrenalin Hydrochloride-Induced Thrombosis in Zebrafish via Regulating Oxidative Stress and Coagulation Cascade.
Liao L, Zhou M, Wang J, Xue X, Deng Y, Zhao X, Peng C, Li Y. Liao L, et al. Front Pharmacol. 2021 Nov 4;12:742954. doi: 10.3389/fphar.2021.742954. eCollection 2021. Front Pharmacol. 2021. PMID: 34803688 Free PMC article. - Cardioprotective Effect of Rumex vesicarius Linn. Leaf Extract against Catecholamine-Induced Cardiotoxicity.
Khan IA, Hussain M, Hussain N, Alqahtani AM, Alqahtani T. Khan IA, et al. Molecules. 2022 May 24;27(11):3383. doi: 10.3390/molecules27113383. Molecules. 2022. PMID: 35684321 Free PMC article. - Stress and the "extended" autonomic system.
Goldstein DS. Goldstein DS. Auton Neurosci. 2021 Dec;236:102889. doi: 10.1016/j.autneu.2021.102889. Epub 2021 Oct 2. Auton Neurosci. 2021. PMID: 34656967 Free PMC article. Review. - A prospective observational study on impact of epinephrine administration route on acute myocardial infarction patients with cardiac arrest in the catheterization laboratory (iCPR study).
Aldujeli A, Haq A, Tecson KM, Kurnickaite Z, Lickunas K, Bailey S, Tatarunas V, Braukyliene R, Baksyte G, Aldujeili M, Khalifeh H, Briedis K, Ordiene R, Unikas R, Hamadeh A, Brilakis ES. Aldujeli A, et al. Crit Care. 2022 Dec 20;26(1):393. doi: 10.1186/s13054-022-04275-8. Crit Care. 2022. PMID: 36539907 Free PMC article. - Potential cellular endocrinology mechanisms underlying the effects of Chinese herbal medicine therapy on asthma.
Meng Z, Chen H, Deng C, Meng S. Meng Z, et al. Front Endocrinol (Lausanne). 2022 Aug 16;13:916328. doi: 10.3389/fendo.2022.916328. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36051395 Free PMC article. Review.
References
- Bolli P., Erne P., Ji B. H., Block L. H., Kiowski W., Bühler F. R. (1984). Adrenaline induces vasoconstriction through post-junctional alpha 2 adrenoceptors and this response is enhanced in patients with essential hypertension. J. Hypertens. Suppl. 2 S115–S118. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources