Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy - PubMed (original) (raw)
Review
. 2021 Jun 1;5(2):021508.
doi: 10.1063/5.0037298. eCollection 2021 Jun.
Affiliations
- PMID: 34104846
- PMCID: PMC8172248
- DOI: 10.1063/5.0037298
Review
Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy
Aleena Mary Cherian et al. APL Bioeng. 2021.
Abstract
Coronary in-stent restenosis and late stent thrombosis are the two major inadequacies of vascular stents that limit its long-term efficacy. Although restenosis has been successfully inhibited through the use of the current clinical drug-eluting stent which releases antiproliferative drugs, problems of late-stent thrombosis remain a concern due to polymer hypersensitivity and delayed re-endothelialization. Thus, the field of coronary stenting demands devices having enhanced compatibility and effectiveness to endothelial cells. Nanotechnology allows for efficient modulation of surface roughness, chemistry, feature size, and drug/biologics loading, to attain the desired biological response. Hence, surface topographical modification at the nanoscale is a plausible strategy to improve stent performance by utilizing novel design schemes that incorporate nanofeatures via the use of nanostructures, particles, or fibers, with or without the use of drugs/biologics. The main intent of this review is to deliberate on the impact of nanotechnology approaches for stent design and development and the recent advancements in this field on vascular stent performance.
© 2021 Author(s).
Figures
FIG. 1.
Various nanoscale surface engineering strategies (nanostructured surface and thin films, nanoparticulate, and nanofibrous) adopted as coatings on coronary bare-metal stents to prevent in-stent restenosis and promote re-endothelialization.
FIG. 2.
(a) Electron micrograph of titania nanotube coated stent. Inset: nanotubes with an average nanotube diameter of 90 nm (magnification ×250 000). Moffat trichrome-stained images of a stented artery. (b) Titania nanoengineered and (c) Ti stents, showing a 15.6% and 5.6% thinner neointima over the struts for TiO2 NT stents than Ti stent. Reprinted with permission from Nuhn_et al._, ACS Appl. Mater. Interfaces 9(23), 19677–19686 (2017). Copyright 2017 American Chemical Society.
FIG. 3.
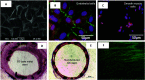
(a) Electron micrograph of titania nanoleafy textured stent surface (magnification ×40 000). Fluorescence imaging of (b) endothelial cells stained for F-actin (red) and PECAM1 (green) and (c) smooth muscle cells stained for F-actin (red) and nucleus (blue) on nanotextured SS surfaces, showing preferential adsorption and proliferation of ECs over SMCs on nanoleafy SS surface. Reprinted with permission from Mohan et al., Adv. Healthcare Mater.6, 1601353 (2017). Copyright 2017 John Wiley and Sons. H&E images of rabbit iliac artery after 2 months implantation of (d) SS bare-metal stent and (e) nanotextured SS stent, showing nearly 50% decrease in neointimal stenosis for the nanotextured stent. (f) Immunofluorescent en-face stained images of wheat germ agglutinin on ECs in nanotextured stent implanted artery, showing complete endothelialization (scale bar: 10 _μ_m). (a) and (d)–(f) Reprinted with permission from Cherian et al., ACS Omega5, 17582–17591 (2020). Copyright 2020 American Chemical Society.
FIG. 4.
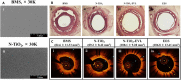
(a) SEM images of (i) BMS and (ii) N–TiO2 film deposited on a bare stent. (b) Histopathological H&E staining and (c) optical coherence tomography of porcine coronary arteries implanted with (i) BMS, (ii) N–TiO2, (iii) N–TiO2 with everolimus, and (iv) EES for 4 weeks. The restenosis area was significantly decreased in the N–TiO2-everolimus group compared to that in the BMS group and was at par with the commercial EES. Reprinted with permission from Park et al., Mater. Sci. Eng.: C 91, 615–623 (2018). Copyright 2018 Elsevier. (BMS: bare-metal stent; N–TiO2: nitrogen-doped TiO2 film; EES: everolimus-eluting stent.)
FIG. 5.
(a) Bare-metal stent with a nanofibrous membrane coating. (b) SEM micrographs of the electrospun nanofibrous membrane with ticagrelor. Magnification of ×3000. (c) SEM images of platelets on electrospun ticagrelor eluting membrane. Red arrow indicates activated platelets (scale bars = 10 _μ_m). Magnification 3000×. (d) Hematoxylin–eosin stained section of arterial lesions in ticagrelor group exhibiting a complete lining of endothelial cells (red arrows). (e) Pathological arterial lesions in the ticagrelor group stained using HES5 markers at 4 weeks following stent implantation. The amount of formed neointima suggests less proliferation of SMCs in the media. (f) SEM images of the stented vessel showing complete endothelial coverage in the ticagrelor group. Reprinted with permission from Lee et al., Int. J. Nanomed. 13, 6039–6048 (2018). Copyright 2018 Dove Medical Press.
FIG. 6.
SEM images of (a) heparin/NONOate nanoparticles immobilized on polyglycidyl methacrylate (PGMA)-coated SS stents. Strut coverage on (b) SS-PGMA-Hep/NONOates and (c) control 316L SS stents harvested at 1 month. Histological hematoxylin−eosin stained images of (d) SS-PGMA-Hep/NONOates and (e) 316L SS stent after implantation for 1 month (arrows point to the higher magnification images). Reprinted with permission from Zhu et al., Langmuir 36, 2901–2910 (2020). Copyright 2020 American Chemical Society.
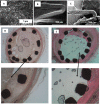
FIG. 7.
Representative SEM images of clinically tested porous stents. (a-i) Microporous hydroxyapatite-coated stent filled with sirolimus formulation (a-ii) cross section of the nanothin hydroxyapatite coating (∼600 nm). Reprinted with permission from Costa_et al._, JACC: Cardiovasc. Interventions 2(5), 422–427 (2008). Copyright 2008, Elsevier. (b-i) Nano+TM polymer-free stent showing strut microstructure after expansion. Strut thickness is approximately 91_μ_m having a large number of sirolimus-filled pores (∼400 nm) on the abluminal stent surface. (b-ii) Electron microscopy images of the nanopores (magnification ×20 000). Reprinted with permission from Liu et al., Catheterization Cardiovasc. Interventions 95(S1), 658–664 (2020). Copyright 2020 John Wiley and Sons.
Similar articles
- Enhancing Stent Effectiveness with Nanofeatures.
Bassous N, Cooke JP, Webster TJ. Bassous N, et al. Methodist Debakey Cardiovasc J. 2016 Sep;12(3):163-168. doi: 10.14797/mdcj-12-3-163. Methodist Debakey Cardiovasc J. 2016. PMID: 27826371 Free PMC article. Review. - Drug-eluting stents.
García-García HM, Vaina S, Tsuchida K, Serruys PW. García-García HM, et al. Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review. - Coupled benefits of nanotopography and titania surface chemistry in fostering endothelialization and reducing in-stent restenosis in coronary stents.
Cherian AM, Joseph J, Nair MB, Nair SV, Vijayakumar M, Menon D. Cherian AM, et al. Biomater Adv. 2022 Nov;142:213149. doi: 10.1016/j.bioadv.2022.213149. Epub 2022 Oct 12. Biomater Adv. 2022. PMID: 36270158 - Are impaired endothelial progenitor cells involved in the processes of late in-stent thrombosis and re-endothelialization of drug-eluting stents?
Zhao FH, Chen YD, Jin ZN, Lu SZ. Zhao FH, et al. Med Hypotheses. 2008;70(3):512-4. doi: 10.1016/j.mehy.2007.05.055. Epub 2007 Aug 30. Med Hypotheses. 2008. PMID: 17764856
Cited by
- Evolution of Coronary Stent Platforms: A Brief Overview of Currently Used Drug-Eluting Stents.
Brami P, Fischer Q, Pham V, Seret G, Varenne O, Picard F. Brami P, et al. J Clin Med. 2023 Oct 24;12(21):6711. doi: 10.3390/jcm12216711. J Clin Med. 2023. PMID: 37959177 Free PMC article. Review. - A Study of PLA Thin Film on SS 316L Coronary Stents Using a Dip Coating Technique.
Macías-Naranjo M, Sánchez-Domínguez M, Rubio-Valle JF, Rodríguez CA, Martín-Alfonso JE, García-López E, Vazquez-Lepe E. Macías-Naranjo M, et al. Polymers (Basel). 2024 Jan 19;16(2):284. doi: 10.3390/polym16020284. Polymers (Basel). 2024. PMID: 38276692 Free PMC article. - Coatings for Cardiovascular Stents-An Up-to-Date Review.
Udriște AS, Burdușel AC, Niculescu AG, Rădulescu M, Grumezescu AM. Udriște AS, et al. Int J Mol Sci. 2024 Jan 16;25(2):1078. doi: 10.3390/ijms25021078. Int J Mol Sci. 2024. PMID: 38256151 Free PMC article. Review. - Drug-Eluting Stents: Technical and Clinical Progress.
Koźlik M, Harpula J, Chuchra PJ, Nowak M, Wojakowski W, Gąsior P. Koźlik M, et al. Biomimetics (Basel). 2023 Feb 9;8(1):72. doi: 10.3390/biomimetics8010072. Biomimetics (Basel). 2023. PMID: 36810403 Free PMC article. Review. - Advances in Use of Nanomaterials for Musculoskeletal Regeneration.
Jampilek J, Placha D. Jampilek J, et al. Pharmaceutics. 2021 Nov 24;13(12):1994. doi: 10.3390/pharmaceutics13121994. Pharmaceutics. 2021. PMID: 34959276 Free PMC article. Review.
References
- Lovely Chhabra A., Zain M. A., and Siddiqui W. J., Coronary Stents ( StatPearls Publishing, Treasure Island, FL, 2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources