Leptomeningeal collateral activation indicates severely impaired cerebrovascular reserve capacity in patients with symptomatic unilateral carotid artery occlusion - PubMed (original) (raw)
Comparative Study
Leptomeningeal collateral activation indicates severely impaired cerebrovascular reserve capacity in patients with symptomatic unilateral carotid artery occlusion
Martina Sebök et al. J Cereb Blood Flow Metab. 2021 Nov.
Abstract
For patients with symptomatic unilateral internal carotid artery (ICA) occlusion, impaired cerebrovascular reactivity (CVR) indicates increased stroke risk. Here, the role of collateral activation remains a matter of debate, whereas angio-anatomical collateral abundancy does not necessarily imply sufficient compensatory flow provided. We aimed to further elucidate the role of collateral activation in the presence of impaired CVR. From a prospective database, 62 patients with symptomatic unilateral ICA occlusion underwent blood oxygenation-level dependent (BOLD) fMRI CVR imaging and a transcranial Doppler (TCD) investigation for primary and secondary collateral activation. Descriptive statistic and multivariate analysis were used to evaluate the relationship between BOLD-CVR values and collateral activation. Patients with activated secondary collaterals exhibited more impaired BOLD-CVR values of the ipsilateral hemisphere (p = 0.02). Specifically, activation of leptomeningeal collaterals showed severely impaired ipsilateral hemisphere BOLD-CVR values when compared to activation of ophthalmic collaterals (0.05 ± 0.09 vs. 0.12 ± 0.04, p = 0.005). Moreover, the prediction analysis showed leptomeningeal collateral activation as a strong independent predictor for ipsilateral hemispheric BOLD-CVR. In our study, ipsilateral leptomeningeal collateral activation is the sole collateral pathway associated with severely impaired BOLD-CVR in patients with symptomatic unilateral ICA occlusion.
Keywords: BOLD fMRI; TCD; carotid artery occlusion; cerebrovascular reactivity; collaterals.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Figure 1.
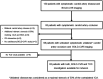
Study flow chart. From 150 patients with symptomatic carotid artery disease included in our prospective BOLD-CVR database, 85 patients were diagnosed with symptomatic carotid artery occlusion. Out of them, 68 patients presented with untreated symptomatic unilateral (maximal stenosis of 50% of the contralateral ICA) carotid artery occlusion and underwent a BOLD-CVR study and were available for inclusion in this prospective cohort study. In six patients out of 68, no TCD study was available. In the final analysis, 62 patients with untreated unilateral symptomatic ICA occlusion, who underwent both, BOLD-CVR and TCD investigation, were included. BOLD: blood oxygen-level dependent; CVR: cerebrovascular reactivity; ICA: internal carotid artery; TCD: transcranial Doppler.
Figure 2.
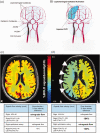
Schematic representation of collateral pathways and exemplary BOLD-CVR images of two patients with right ICA occlusion. (a) The figure shows the available four collateral pathways (two primary collaterals and two secondary collaterals) in patients with unilateral ICA occlusion. The two primary collaterals, which are part of the Circle of Willis, are ACOM and PCOM. Activation of ACOM is detected as reversed flow direction in the first (A1) segment of the anterior cerebral artery ipsilateral to the occluded ICA. Activation of PCOM is identified as cerebral blood flow increase of > 50% in the ipsilateral first (P1) segment of the posterior cerebral artery compared with the contralateral side. The two TCD-derived secondary collaterals are ophthalmic and leptomeningeal collaterals supplied by the posterior circulation. Activation of ophthalmic collaterals is detected as reversed flow in the periorbital arteries and activation of leptomeningeal collaterals as flow increase of > 30% in the ipsilateral second (P2) segment of posterior cerebral artery compared with the contralateral P2 segment. (B) The figure shows activation of leptomeningeal collaterals on the surface of the ipsilateral (=side of ICA occlusion) hemisphere with impaired (=paradox) CVR in this hemisphere. (c) BOLD-CVR image of a 48-years old patient with occlusion of right ICA showed preserved CVR in the territory of the occluded vessel. The TCD examination showed only primary activation through ACOM with reversed flow direction in the right ACA-A1 segment. (d) BOLD-CVR image of 85-years old patient with occlusion of right ICA showed impaired CVR in the territory of the occluded vessel. The TCD examination showed: 1) right sided PCOM activation with 184% increase of SFV of the right PCA-P1 segment compared to the contralateral PCA-P1, 2) reversed flow in the right ophthalmic artery indicating right sided ophthalmic activation, and 3) activation of leptomeningeal collateral pathways supplied by the posterior circulation on the ride side with 130% increase of SFV of the right PCA-P2 segment compared to the contralateral PCA-P2. ACA-A1: first segment of anterior cerebral artery; ACOM: anterior communicating artery; BOLD: blood oxygenation-level dependent; CVR: cerebrovascular reactivity; ICA: internal carotid artery; PCA-P1: first segment of posterior cerebral artery; PCA-P2: second segment of posterior cerebral artery; PCOM: posterior communicating artery; SFV: systolic flow velocity; TCD: transcranial Doppler.
Figure 3.
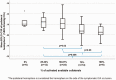
Correlation between percentage of activated available collaterals and mean ipsilateral hemisphere BOLD-CVR values. Box-whisker plots show the correlation between the percentages of activated available collaterals and mean BOLD-CVR values of the ipsilateral hemisphere. Patients with more activated collaterals exhibit significantly lower CVR values: patients with 75% and 100% of activated available collaterals have significantly lower mean BOLD-CVR of ipsilateral hemisphere as compared to patients with activated 25-33% of available collaterals; patients with 100% collateral activation exhibit significantly lower CVR values compared to patients with 50-67% collateral activation. Activation of maximal four collateral pathways (ACOM, PCOM, ophthalmic artery, and leptomeningeal collateral pathways supplied by the posterior circulation) is possible. However, after anatomical correction there are also patients with only three or two anatomically possible collaterals. Therefore, the quotient between activated collaterals in TCD and possible collaterals in anatomical images is always calculated for the corrected number of anatomical collaterals. For example: in patient with three possible collaterals as seen in TOF MRA and activation of two of those collaterals in TCD, the percentage of activated collaterals is calculated as: 2/3 * 100% = 67%. Patients with activation of 1/4 and 1/3 of anatomically available collaterals form the 25-33% group and patients with activation of 1/2 and 2/3 of anatomically available the 50-67% group. Note: The box of box-whisker plots represents the median value with interquartile range (25th to the 75th percentile). The upper and lower whiskers represent values outside the middle 50% (i.e., the values below 25th and above 75th percentile). BOLD: blood oxygen-level dependent; CVR: cerebrovascular reactivity.
Figure 4.
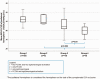
Correlation between different stages of secondary collaterals activation and mean ipsilateral hemisphere BOLD-CVR values. Box-whisker plots show the correlation between the three groups of patients with different secondary collaterals activation status and BOLD-CVR values of ipsilateral hemisphere. Patients with activated leptomeningeal collateral pathways supplied by the posterior circulation with/without activation of ophthalmic collaterals (group 3) exhibit significantly lower mean BOLD-CVR values of ipsilateral hemisphere compared to patients without any secondary collaterals (group 1) (mean BOLD-CVR ± SD: 0.05±0.09 vs. 0.13±0.05, p=0.003) as well as compared to patients with activation of only ophthalmic collaterals (group 2) (mean BOLD-CVR ± SD: 0.05±0.09 vs. 0.12±0.04, p=0.005). No difference is BOLD-CVR values is discernible between patients without any activated secondary collaterals and between patients with only ophthalmic activation. The between groups difference for ipsilateral BOLD-CVR values by one-way ANOVA is p=0.001. Note: The box of box-whisker plots represents the median value with interquartile range (25th to the 75th percentile). The upper and lower whiskers represent values outside the middle 50% (i.e., the values below 25th and above 75th percentile). ANOVA: analysis of variance; BOLD: blood oxygen-level dependent; CVR: cerebrovascular reactivity; SD: standard deviation.
Figure 5.
Correlation between different stages of PCOM and leptomeningeal collaterals activation and mean ipsilateral hemisphere BOLD-CVR values. Box-whisker plots show the correlation between the groups of patients with different activation status of PCOM and leptomeningeal collateral pathways supplied by the posterior circulation and BOLD-CVR values of ipsilateral hemisphere. Patients with activated both, PCOM and leptomeningeal collaterals (group 4) exhibit significantly lower mean BOLD-CVR values of ipsilateral hemisphere compared to patients with only PCOM activation (group 2) (mean BOLD-CVR ± SD: -0.01±0.06 vs. 0.12±0.06, p=0.003) and patients with only leptomeningeal activation (group 3) (mean BOLD-CVR ± SD: mean BOLD-CVR ± SD: -0.01±0.06 vs. 0.07±0.09, p=0.03). The between groups difference for ipsilateral BOLD-CVR values by one-way ANOVA is p<0.001. Note: The box of box-whisker plots represents the median value with interquartile range (25th to the 75th percentile). The upper and lower whiskers represent values outside the middle 50% (i.e., the values below 25th and above 75th percentile). ANOVA: analysis of variance; BOLD: blood oxygen-level dependent; CVR: cerebrovascular reactivity; PCOM: posterior communicating artery; SD: standard deviation.
Similar articles
- Reduced contralateral cerebrovascular reserve in patients with unilateral steno-occlusive disease.
Sam K, Small E, Poublanc J, Han JS, Mandell DM, Fisher JA, Crawley AP, Mikulis DJ. Sam K, et al. Cerebrovasc Dis. 2014;38(2):94-100. doi: 10.1159/000362084. Epub 2014 Oct 2. Cerebrovasc Dis. 2014. PMID: 25277683 - Mapping Cerebrovascular Reactivity Impairment in Patients With Symptomatic Unilateral Carotid Artery Disease.
Sebök M, van Niftrik CHB, Winklhofer S, Wegener S, Esposito G, Stippich C, Luft A, Regli L, Fierstra J. Sebök M, et al. J Am Heart Assoc. 2021 Jun 15;10(12):e020792. doi: 10.1161/JAHA.121.020792. Epub 2021 Jun 9. J Am Heart Assoc. 2021. PMID: 34102856 Free PMC article. - Predictors of poor cerebral collaterals and cerebrovascular reserve in patients with chronic total carotid occlusion.
Rizk H, Allam M, Hegazy A, Khalil H, Helmy H, Hashem HS, Abd-Allah F. Rizk H, et al. Int J Neurosci. 2019 May;129(5):455-460. doi: 10.1080/00207454.2018.1538990. Epub 2018 Nov 27. Int J Neurosci. 2019. PMID: 30372650 - Arterial Spin Labeling and Blood Oxygen Level-Dependent MRI Cerebrovascular Reactivity in Cerebrovascular Disease: A Systematic Review and Meta-Analysis.
Smeeing DP, Hendrikse J, Petersen ET, Donahue MJ, de Vis JB. Smeeing DP, et al. Cerebrovasc Dis. 2016;42(3-4):288-307. doi: 10.1159/000446081. Epub 2016 May 31. Cerebrovasc Dis. 2016. PMID: 27237626 Review. - Leptomeningeal anastomoses: Mechanisms of pial collateral remodeling in ischemic stroke.
Kaloss AM, Theus MH. Kaloss AM, et al. WIREs Mech Dis. 2022 Jul;14(4):e1553. doi: 10.1002/wsbm.1553. Epub 2022 Feb 3. WIREs Mech Dis. 2022. PMID: 35118835 Free PMC article. Review.
Cited by
- Early Cerebral Microvasculature Impairment and Increased Body Mass Index in Patients with Psoriasis.
Piec K, Marek-Józefowicz L, Nadolska K, Lemanowicz A, Serafin Z, Kozera G. Piec K, et al. Biomedicines. 2024 Jul 23;12(8):1627. doi: 10.3390/biomedicines12081627. Biomedicines. 2024. PMID: 39200092 Free PMC article. - BOLD Cerebrovascular Reactivity and NOVA Quantitative MR Angiography in Adult Patients with Moyamoya Vasculopathy Undergoing Cerebral Bypass Surgery.
Garbani Nerini L, Bellomo J, Höbner LM, Stumpo V, Colombo E, van Niftrik CHB, Schubert T, Kulcsár Z, Wegener S, Luft A, Regli L, Fierstra J, Sebök M, Esposito G. Garbani Nerini L, et al. Brain Sci. 2024 Jul 29;14(8):762. doi: 10.3390/brainsci14080762. Brain Sci. 2024. PMID: 39199456 Free PMC article. - Association of Carotid Artery Disease with Collateralization and Infarct Growth in Patients with Acute Middle Cerebral Artery Occlusion.
Güney R, Potreck A, Neuberger U, Schmitt N, Purrucker J, Möhlenbruch MA, Bendszus M, Seker F. Güney R, et al. AJNR Am J Neuroradiol. 2024 May 9;45(5):574-580. doi: 10.3174/ajnr.A8180. AJNR Am J Neuroradiol. 2024. PMID: 38575322 - Flow-augmentation STA-MCA bypass for acute and subacute ischemic stroke due to internal carotid artery occlusion and the role of advanced neuroimaging with hemodynamic and flow-measurement in the decision-making: preliminary data.
Sebök M, Höbner LM, Fierstra J, Schubert T, Wegener S, Kulcsár Z, Luft AR, Regli L, Esposito G. Sebök M, et al. Quant Imaging Med Surg. 2024 Jan 3;14(1):777-788. doi: 10.21037/qims-23-876. Epub 2024 Jan 2. Quant Imaging Med Surg. 2024. PMID: 38223058 Free PMC article. - Hemodynamic Failure Staging With Blood Oxygenation Level-Dependent Cerebrovascular Reactivity and Acetazolamide-Challenged (15O-)H2O-Positron Emission Tomography Across Individual Cerebrovascular Territories.
Sebök M, van der Wouden F, Mader C, Pangalu A, Treyer V, Fisher JA, Mikulis DJ, Hüllner M, Regli L, Fierstra J, van Niftrik CHB. Sebök M, et al. J Am Heart Assoc. 2023 Dec 19;12(24):e029491. doi: 10.1161/JAHA.123.029491. Epub 2023 Dec 12. J Am Heart Assoc. 2023. PMID: 38084716 Free PMC article.
References
- Flaherty ML, Flemming KD, McClelland R, et al.. Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke 2004; 35: e349-52. - PubMed
- Papassin J, Heck O, Condamine E, et al.. Impaired cerebrovascular reactivity is associated with recurrent stroke in patients with severe intracranial arterial stenosis: a C02 BOLD fMRI study. J Neuroradiol 2020; S0150-9861(20)30165-6. - PubMed
- Müller M, andSchimrigk K.. Vasomotor reactivity and pattern of collateral blood flow in severe occlusive carotid artery disease. Stroke 1996; 27: 296–299. - PubMed
- Schomer DF, Marks MP, Steinberg GK, et al.. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994; 330: 1565–1570. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous