Advancing to the era of cancer immunotherapy - PubMed (original) (raw)
Review
. 2021 Sep;41(9):803-829.
doi: 10.1002/cac2.12178. Epub 2021 Jun 24.
Affiliations
- PMID: 34165252
- PMCID: PMC8441060
- DOI: 10.1002/cac2.12178
Review
Advancing to the era of cancer immunotherapy
Yun Wang et al. Cancer Commun (Lond). 2021 Sep.
Abstract
Cancer greatly affects the quality of life of humans worldwide and the number of patients suffering from it is continuously increasing. Over the last century, numerous treatments have been developed to improve the survival of cancer patients but substantial progress still needs to be made before cancer can be truly cured. In recent years, antitumor immunity has become the most debated topic in cancer research and the booming development of immunotherapy has led to a new epoch in cancer therapy. In this review, we describe the relationships between tumors and the immune system, and the rise of immunotherapy. Then, we summarize the characteristics of tumor-associated immunity and immunotherapeutic strategies with various molecular mechanisms by showing the typical immune molecules whose antibodies are broadly used in the clinic and those that are still under investigation. We also discuss important elements from individual cells to the whole human body, including cellular mutations and modulation, metabolic reprogramming, the microbiome, and the immune contexture. In addition, we also present new observations and technical advancements of both diagnostic and therapeutic methods aimed at cancer immunotherapy. Lastly, we discuss the controversies and challenges that negatively impact patient outcomes.
Keywords: adverse effects; cancer; hyperprogressive disease; immune checkpoints; immunity; immunotherapy; metabolic reprogramming; microbiome; mutation.
© 2021 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
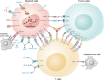
FIGURE 1
Variable interactions among immune checkpoints in the TME. There are many immune checkpoints in the TME. Some of them are expressed mainly on T cells including PD‐1, CTLA‐4, and LAG‐3, which could suppress the function of CTLs. The others are mainly expressed on myeloid cells, such as c‐Rel and MerTK, which could enhance the inhibitory function of MDSCs to tumor cells. Abbreviations: TME: tumor microenvironment; CTLs: cytotoxic T lymphocytes; IL: interleukin; CD: cluster of differentiation; MHC: major histocompatibility complex; PD‐1: programmed cell death‐1; PD‐L1: programmed cell death‐Ligand 1; PD‐L2: programmed cell death‐Ligand 2; CTLA‐4: cytotoxic T lymphocyte‐associated antigen 4; VISTA: V‐domain immunoglobulin suppressor of T cell activation; TIGIT: T cell immunoglobulin and ITIM domain; TIM‐3: T cell immunoglobulin and mucin domain‐containing protein 3; LAG‐3: lymphocyte activation gene‐3; ATP: adenosine triphosphate; AMP: adenosine monophosphate; GPI: glycosylphosphatidylinositol; SIRPα: signal regulatory protein α; SIGLEC‐15: sialic acid binding Ig‐like lectin 15; GM‐CSF: granulocyte‐macrophage colony stimulating factor; IL‐RAcP: interleukin‐1 receptor accessory protein; ST2: suppression of tumorigenicity 2; MERTK: c‐mer proto‐oncogene tyrosine kinase; GAS6: growth arrest specific 6; PtdSer: phosphatidylserine; TCR: T cell receptor; SIGLEC‐10: sialic acid binding Ig‐like lectin 10; VSIG‐3: V‐set and immunoglobulin domain‐containing protein 3; LGALS9: galectin‐9; DAP12: DNAX‐activation protein 12; sTn: sialyl‐Tn; PI3K: phosphoinositide 3‐kinase; ARG1: arginase‐1; NOS2: nitric oxide synthase 2
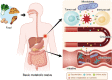
FIGURE 2
Metabolic interactions in the TME are based on the basic status of the patient. Food is digested and decomposed into metabolites and nutrients based on the basic metabolic status of the human body. The intestinal microbiome participates in the metabolism of these small molecules and influences their levels in the blood. Then, the metabolites and nutrients are sent to the tumor site, forming a competition for nutrients between tumor cells and immune cells in the TME, which is also affected by the local microbiome. Abbreviations: TME: tumor microenvironment
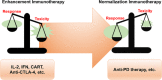
FIGURE 3
The balance between response and toxicity in anti‐PD therapy compared with other immunotherapy methods. Toxicity outweighs the response in immunotherapy methods such as IL‐2, IFNs, CAR‐T cells, and anti‐CTLA‐4 antibodies, resulting in quite limited indications. For anti‐PD therapy, the response outweighs toxicity considerably, which leads to a rather broad application. Abbreviations: CAR‐T: chimeric antigen receptor T cells; CTLA‐4: cytotoxic T lymphocyte‐associated antigen 4; IFN: interferon; IL‐2: interleukin‐2; PD: programmed death
FIGURE 4
Adding a limited course of chemotherapy to immunotherapy to address HPD. Accelerated disease progression due to HPD significantly compromised the total survival benefit of anti‐PD therapy over chemotherapy, which could be rescued by adding a limited course of chemotherapy to anti‐PD therapy. Abbreviations: HPD: hyperprogressive disease; PD: programmed death
Similar articles
- The New Era of Cancer Immunotherapy: Manipulating T-Cell Activity to Overcome Malignancy.
Khalil DN, Budhu S, Gasmi B, Zappasodi R, Hirschhorn-Cymerman D, Plitt T, De Henau O, Zamarin D, Holmgaard RB, Murphy JT, Wolchok JD, Merghoub T. Khalil DN, et al. Adv Cancer Res. 2015;128:1-68. doi: 10.1016/bs.acr.2015.04.010. Epub 2015 Jun 9. Adv Cancer Res. 2015. PMID: 26216629 Review. - Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy.
Guerra L, Bonetti L, Brenner D. Guerra L, et al. Cell Rep. 2020 Jul 7;32(1):107848. doi: 10.1016/j.celrep.2020.107848. Cell Rep. 2020. PMID: 32640218 Review. - Harnessing Metabolic Reprogramming to Improve Cancer Immunotherapy.
Yan L, Tan Y, Chen G, Fan J, Zhang J. Yan L, et al. Int J Mol Sci. 2021 Sep 24;22(19):10268. doi: 10.3390/ijms221910268. Int J Mol Sci. 2021. PMID: 34638609 Free PMC article. Review. - Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects.
Jia H, Truica CI, Wang B, Wang Y, Ren X, Harvey HA, Song J, Yang JM. Jia H, et al. Drug Resist Updat. 2017 May;32:1-15. doi: 10.1016/j.drup.2017.07.002. Epub 2017 Aug 19. Drug Resist Updat. 2017. PMID: 29145974 Review. - Immunometabolism: A new target for improving cancer immunotherapy.
Guo C, Chen S, Liu W, Ma Y, Li J, Fisher PB, Fang X, Wang XY. Guo C, et al. Adv Cancer Res. 2019;143:195-253. doi: 10.1016/bs.acr.2019.03.004. Epub 2019 Apr 17. Adv Cancer Res. 2019. PMID: 31202359 Free PMC article. Review.
Cited by
- Canadian National Pancreas Conference 2023: A Review of Multidisciplinary Engagement in Pancreatic Cancer Care.
Nickerson JL, Cyr C, Arseneau RJ, Lee SN, Condon-Oldreive S, Zogopoulos G, Roberts K, Kim CA, Ng SSW, Haider M, Villalba E, Stephenson L, Tsang E, Johnston B, Gala-Lopez B, Cooper V, Hannon B, Gangloff A, Gill S, Servidio-Italiano F, Ramjeesingh R. Nickerson JL, et al. Curr Oncol. 2024 Oct 16;31(10):6191-6204. doi: 10.3390/curroncol31100461. Curr Oncol. 2024. PMID: 39451765 Free PMC article. Review. - Machine learning-based model for CD4+ conventional T cell genes to predict survival and immune responses in colorectal cancer.
Wang Z, Sun Z, Lv H, Wu W, Li H, Jiang T. Wang Z, et al. Sci Rep. 2024 Oct 18;14(1):24426. doi: 10.1038/s41598-024-75270-y. Sci Rep. 2024. PMID: 39424871 Free PMC article. - Targeting VPS18 hampers retromer trafficking of PD-L1 and augments immunotherapy.
Dong T, Niu H, Chu Z, Zhou C, Gao Y, Jia M, Sun B, Zheng X, Zhang W, Zhang J, Luo Y, Sun Y, Wang C, Lu Q, Liu C, Shao G, Lou H, Yuan H. Dong T, et al. Sci Adv. 2024 Oct 18;10(42):eadp4917. doi: 10.1126/sciadv.adp4917. Epub 2024 Oct 16. Sci Adv. 2024. PMID: 39413192 Free PMC article. - Nanomedicines Targeting Tumor Cells or Tumor-Associated Macrophages for Combinatorial Cancer Photodynamic Therapy and Immunotherapy: Strategies and Influencing Factors.
Wei Y, Li R, Wang Y, Fu J, Liu J, Ma X. Wei Y, et al. Int J Nanomedicine. 2024 Oct 4;19:10129-10144. doi: 10.2147/IJN.S466315. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39381025 Free PMC article. Review. - PAK4 Is Involved in the Stabilization of PD-L1 and the Resistance to Doxorubicin in Osteosarcoma and Predicts the Survival of Diagnosed Patients.
Zhang J, Song Y, Ahn AR, Park HS, Park SH, Moon YJ, Kim KM, Jang KY. Zhang J, et al. Cells. 2024 Aug 28;13(17):1444. doi: 10.3390/cells13171444. Cells. 2024. PMID: 39273017 Free PMC article.
References
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor‐infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–66. - PubMed
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources