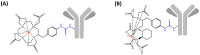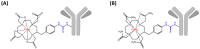Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The "Hopeful Eight" - PubMed (original) (raw)
Review
Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The "Hopeful Eight"
Romain Eychenne et al. Pharmaceutics. 2021.
Abstract
Among all existing radionuclides, only a few are of interest for therapeutic applications and more specifically for targeted alpha therapy (TAT). From this selection, actinium-225, astatine-211, bismuth-212, bismuth-213, lead-212, radium-223, terbium-149 and thorium-227 are considered as the most suitable. Despite common general features, they all have their own physical characteristics that make them singular and so promising for TAT. These radionuclides were largely studied over the last two decades, leading to a better knowledge of their production process and chemical behavior, allowing for an increasing number of biological evaluations. The aim of this review is to summarize the main properties of these eight chosen radionuclides. An overview from their availability to the resulting clinical studies, by way of chemical design and preclinical studies is discussed.
Keywords: actinium-225; astatine-211; bismuth-212; bismuth-213; lead-212; radium-223; targeted alpha therapy; terbium-149; thorium-227; α-emitters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 4
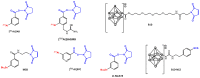
Structures of main prosthetic groups used for 211At radiolabeling. [211At]SAB: _N_-succinimidyl-3-[211At]astatobenzoate [111,113]; [211At]SAGMB: _N_-succinimidyl 3-[211At]astato-4-guanidinomethyl benzoate [123]; B10: maleimido-_closo_-decaborate(2-) derivative [110]; MSB: _N_-2-(maleimido)ethyl-3-(trimethylstannyl)benzamide [115]; [211At]SPC: _N_-succinimidyl 5-[211At]astato-3-pyridinecarboxylate [127]; _m_-MeATE: _N_-succinimidyl-3-(trimethylstannyl)-benzoate [114]; B10-NCS: isothiocyanatophenyl-_closo_-decaborate(2-) derivative [110].
Figure 1
Decay schemes for the production of 225Ac and 213Bi.
Figure 2
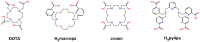
Chemical structures of main 225Ac chelators.
Figure 3
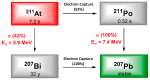
Simplified decay scheme of 211At.
Figure 5
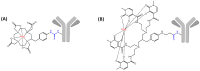
Schematic representation of 213Bi-labeled radioimmunoconjugates based on _p_-SCN-Bn-DOTA (A) or _p_-SCN-Bn-CHX-A″-DTPA (B).
Figure 6
Decay schemes for the production of 212Bi and 212Pb.
Figure 7
Schematic representation of 212Pb-labeled radioimmunoconjugates based on _p_-SCN-Bn-DOTA (A) or _p_-SCN-Bn-TCMC (B).
Figure 8
Decay schemes for the production of 223Ra and 227Th.
Figure 9
Decay scheme of 149Tb.
Figure 10
Schematic representation of 227Th-labeled radioimmunoconjugates based on _p_-SCN-Bn-DOTA (A) or Me-3,2-HOPO (B).
Similar articles
- Radium-223 dichloride in prostate cancer: proof of principle for the use of targeted alpha treatment in clinical practice.
Dizdarevic S, McCready R, Vinjamuri S. Dizdarevic S, et al. Eur J Nucl Med Mol Imaging. 2020 Jan;47(1):192-217. doi: 10.1007/s00259-019-04475-5. Epub 2019 Aug 30. Eur J Nucl Med Mol Imaging. 2020. PMID: 31471713 - Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications.
Nelson BJB, Andersson JD, Wuest F. Nelson BJB, et al. Pharmaceutics. 2020 Dec 31;13(1):49. doi: 10.3390/pharmaceutics13010049. Pharmaceutics. 2020. PMID: 33396374 Free PMC article. Review. - An Appendix of Radionuclides Used in Targeted Alpha Therapy.
Ferrier MG, Radchenko V. Ferrier MG, et al. J Med Imaging Radiat Sci. 2019 Dec;50(4 Suppl 1):S58-S65. doi: 10.1016/j.jmir.2019.06.051. Epub 2019 Aug 16. J Med Imaging Radiat Sci. 2019. PMID: 31427258 Review. No abstract available. - Advancements in cancer therapy with alpha-emitters: a review.
Imam SK. Imam SK. Int J Radiat Oncol Biol Phys. 2001 Sep 1;51(1):271-8. doi: 10.1016/s0360-3016(01)01585-1. Int J Radiat Oncol Biol Phys. 2001. PMID: 11516878 Review. - Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators.
Kozempel J, Mokhodoeva O, Vlk M. Kozempel J, et al. Molecules. 2018 Mar 5;23(3):581. doi: 10.3390/molecules23030581. Molecules. 2018. PMID: 29510568 Free PMC article. Review.
Cited by
- Efficient Production of the PET Radionuclide 133La for Theranostic Purposes in Targeted Alpha Therapy Using the 134Ba(p,2n)133La Reaction.
Brühlmann SA, Kreller M, Pietzsch HJ, Kopka K, Mamat C, Walther M, Reissig F. Brühlmann SA, et al. Pharmaceuticals (Basel). 2022 Sep 21;15(10):1167. doi: 10.3390/ph15101167. Pharmaceuticals (Basel). 2022. PMID: 36297279 Free PMC article. - Where do we stand with radioimmunotherapy for acute myeloid leukemia?
Walter RB. Walter RB. Expert Opin Biol Ther. 2022 May;22(5):555-561. doi: 10.1080/14712598.2022.2060735. Epub 2022 Mar 31. Expert Opin Biol Ther. 2022. PMID: 35350938 Free PMC article. - Somatostatin and Somatostatin Receptors: From Signaling to Clinical Applications in Neuroendocrine Neoplasms.
Del Olmo-Garcia MI, Prado-Wohlwend S, Andres A, Soriano JM, Bello P, Merino-Torres JF. Del Olmo-Garcia MI, et al. Biomedicines. 2021 Dec 1;9(12):1810. doi: 10.3390/biomedicines9121810. Biomedicines. 2021. PMID: 34944626 Free PMC article. Review. - Can current preclinical strategies for radiopharmaceutical development meet the needs of targeted alpha therapy?
Kleynhans J, Ebenhan T, Cleeren F, Sathekge MM. Kleynhans J, et al. Eur J Nucl Med Mol Imaging. 2024 Jun;51(7):1965-1980. doi: 10.1007/s00259-024-06719-5. Epub 2024 Apr 27. Eur J Nucl Med Mol Imaging. 2024. PMID: 38676735 Free PMC article. Review. - Complexation of 3p-_C_-NETA with radiometal ions: A density functional theory study for targeted radioimmunotherapy.
Ramdhani D, Watabe H, Hardianto A, Janitra RS. Ramdhani D, et al. Heliyon. 2024 Jul 20;10(15):e34875. doi: 10.1016/j.heliyon.2024.e34875. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39144950 Free PMC article.
References
- Kassis A.I., Adelstein S.J. Radiobiologic Principles in Radionuclide Therapy. J. Nucl. Med. 2005;46:4S. - PubMed
- Sgouros G., Roeske J.C., McDevitt M.R., Palm S., Allen B.J., Fisher D.R., Brill A.B., Song H., Howell R.W., Akabani G., et al. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of -Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010;51:311–328. doi: 10.2967/jnumed.108.058651. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources