Sphingolipids in metabolic disease: The good, the bad, and the unknown - PubMed (original) (raw)
Review
Sphingolipids in metabolic disease: The good, the bad, and the unknown
Christopher D Green et al. Cell Metab. 2021.
Abstract
The bioactive sphingolipid metabolites ceramide and sphingosine-1-phosphate (S1P) are a recent addition to the lipids accumulated in obesity and have emerged as important molecular players in metabolic diseases. Here we summarize evidence that dysregulation of sphingolipid metabolism correlates with pathogenesis of metabolic diseases in humans. This review discusses the current understanding of how ceramide regulates signaling and metabolic pathways to exacerbate metabolic diseases and the Janus faces for its further metabolite S1P, the kinases that produce it, and the multifaceted and at times opposing actions of S1P receptors in various tissues. Gaps and limitations in current knowledge are highlighted together with the need to further decipher the full array of their actions in tissue dysfunction underlying metabolic pathologies, pointing out prospects to move this young field of research toward the development of effective therapeutics.
Keywords: Ceramide; metabolic diseases; sphingolipid metabolites; sphingosine-1-phosphate.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
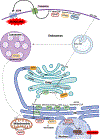
Figure 1.. Spatial distribution and compartmentalization of sphingolipid metabolism.
De novo sphingolipid synthesis begins at the ER as described in the text, leading to the formation of ceramide (Cer). Cer is transported to the Golgi either by the ceramide transfer protein CERT at ER-trans-Golgi contact sites for the formation of sphingomyelin (SM), or by vesicular transport to the cis-Golgi. There, Cer is glucosylated to GluCer, which is then trafficked further in the Golgi via FAPP2 to form LacCer, and sequentially glycosylated to form complex glycosphingolipids (GSLs). Cer may also be translocated to mitochondria at membrane contact sites with the ER. At the plasma membrane, in a signal-mediated process, sphingomyelinase (SMase), ceramidase (CDase), and sphingosine kinases (SphK) produce the bioactive metabolites Cer, sphingosine (Sph) and sphingosine-1-phosphate (S1P), respectively. S1P is then transported outside of cells and acts in a paracrine or autocrine fashion via S1P receptors (S1PRs) to initiate myriad signaling pathways. Plasma membrane sphingolipids are internalized by the endocytic pathway to the lysosome for degradation to Sph, which can be trafficked by unknown mechanisms to the ER. In the ER, Sph can either be recycled back to Cer for reutilization or degraded after phosphorylation by SphKs and cleavage by S1P lyase (SGPL1). S1P produced by SphK2 in mitochondria interacts with the electron transport chain, and in the nucleus, it regulates histone acetylation. Color code: Red box, enzymes; orange box, lipid binding and transport proteins; blue arrows, vesicular transport steps; red arrows, protein-mediated lipid transport step; dashed arrows, unknown transport step(s).
Figure 2.. Ceramide and S1P are critical links in the development of metabolic disease.
Multiple factors associated with increased susceptibility to metabolic dysfunction influence the synthesis of ceramides and S1P. Subsequent derangements in ceramide/S1P levels have significant consequences to glucose and lipid homeostasis in metabolic tissues and the heart and vasculature systems.
Figure 3.. Ceramide modulation and tissue-specific effects on cellular metabolism.
Ceramides generated during the progression of metabolic disease activate protein phosphatase 2A (PP2A) and protein kinase Cζ (PKCζ), which inhibit AKT activation and reduce insulin signaling and glucose uptake. (A) Hepatic ceramides also reduce mitochondrial function and increase lipid accumulation. (B) Adipose tissue ceramides are also linked to decreased fatty acid β-oxidation and lipolysis. (C) In muscle, excess ceramides inhibit expression of the myokine FGF21, a key regulator of glucose metabolism. White boxes highlight the sphingolipid biosynthetic enzymes shown to affect metabolic outcomes when altered in vivo.
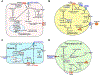
Figure 4.. S1P/S1PR signaling modulation in metabolic tissues.
Regulation of S1P/S1PR signaling exhibits tissue-specific metabolic outcomes. (A) S1P generated in liver hepatocytes can activate AKT to enhance insulin signaling and triglyceride storage as well as regulate sterol, lipid, and inflammatory gene expression. (B) S1P and S1P/S1PR signaling modulates adipocytes inflammation, differentiation, and pathways affecting lipolysis, glucose uptake, and mitochondrial biogenesis. (C) In muscles, S1P/S1PR affects insulin signaling in part through IL6 generation and receptor activation to block AKT activity. (D) In pancreatic β-cells, S1P enhances insulin secretion, and activation of S1PRs suppresses apoptosis and regulates mitochondrial biogenesis.
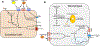
Figure 5.. S1P/S1PR signaling in inflammation and metabolic dysregulation.
(A) Endothelial S1PRs influence inflammation and increase vasodilation through eNOS activation, counteracting effects mediated by ceramides. (B) In macrophages, intracellular S1P enhances degradation of stored lipids, while S1P/S1PR signals block apoptosis and increase ABCA1-mediated cholesterol export. White boxes highlight the sphingolipid biosynthetic enzymes shown to affect metabolic outcomes when altered in vivo.
References
- Al Fadel F, Fayyaz S, Japtok L, and Kleuser B (2016). Involvement of Sphingosine 1-Phosphate in Palmitate-Induced Non-Alcoholic Fatty Liver Disease. Cell Physiol. Biochem 40, 1637–1645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials