Role of the Gut Microbiota in Regulating Non-alcoholic Fatty Liver Disease in Children and Adolescents - PubMed (original) (raw)
Review
Role of the Gut Microbiota in Regulating Non-alcoholic Fatty Liver Disease in Children and Adolescents
Daisuke Tokuhara. Front Nutr. 2021.
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in children and adolescents. Although obesity is the leading cause of NAFLD, the etiologies of NAFLD are multifactorial (e.g., high-fat diet, a lack of exercise, gender, maternal obesity, the antibiotic use), and each of these factors leads to dysbiosis of the gut microbiota community. The gut microbiota is a key player in the development and regulation of the gut mucosal immune system as well as the regulation of both NAFLD and obesity. Dysbiosis of the gut microbiota promotes the development of NAFLD via alteration of gut-liver homeostasis, including disruption of the gut barrier, portal transport of bacterial endotoxin (lipopolysaccharide) to the liver, altered bile acid profiles, and decreased concentrations of short-chain fatty acids. In terms of prevention and treatment, conventional approaches (e.g., dietary and exercise interventions) against obesity and NAFLD have been confirmed to recover the dysbiosis and dysbiosis-mediated altered metabolism. In addition, increased understanding of the importance of gut microbiota-mediated homeostasis in the prevention of NAFLD suggests the potential effectiveness of gut microbiota-targeted preventive and therapeutic strategies (e.g., probiotics and fecal transplantation) against NAFLD in children and adolescents. This review comprehensively summarizes our current knowledge of the gut microbiota, focusing on its interaction with NAFLD and its potential therapeutic role in obese children and adolescents with this disorder.
Keywords: bile acid; children; dysbiosis; gut microbiota; lipopolysaccharide; non-alcoholic fatty liver disease; short-chain fatty acid.
Copyright © 2021 Tokuhara.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
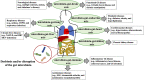
Figure 1
Interaction between dysbiosis or disruption of the gut microbial community and human diseases. Dysbiosis or disruption of the gut microbiota composition that is induced primarily through an imbalanced lifestyle (e.g., high-fat, low-fiber diet) leads to the disruption of the gut barrier, increased gut permeability, systemic circulation of bacterial endotoxins [e.g., lipopolysaccharide (LPS)] to multiple organs (e.g., liver, brain, kidney) and functional systems (e.g., immune system, endocrine systems), and altered production of beneficial metabolites [e.g., short-chain fatty acids (SCFAs)] by normal gut microbiota. Bidirectional and unfavorable crosstalk among the dysbiotic microbiota, gastrointestinal system, and target organs and functional systems (e.g., microbiota-gut-liver axis, microbiota-gut-brain axis) is involved in the pathogenesis of the various diseases (e.g., NAFLD, chronic kidney diseases, Alzheimer's disease, asthma) of the host. COPD, chronic obstructive pulmonary disease; NAFLD, non-alcoholic fatty liver disease; GI, gastrointestinal; SLE, systemic lupus erythematosus; HBV, hepatitis B virus; HCV, hepatitis C virus.
Figure 2
Gut microbiota in children. The gut microbiota demonstrates age-dependent increases in diversity and changes in composition (i.e., relative high abundance of Bifidobacteria throughout childhood). These gut microbiota profiles in children are influenced by various life events (e.g., mode of delivery, weaning, and infections), life style choices (e.g., exercise habits, dietary patterns, sleep hygiene), ethnicity, and gender.
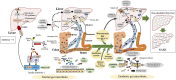
Figure 3
Mechanisms of dysbiosis-induced NAFLD in children and adolescents. Dysbiosis and disruption of the gut microbiota contribute to the development of non-alcoholic fatty liver disease (NAFLD) via modulation of the gut–liver homeostasis, including the involvement of the gut barrier, bacterial endotoxin [lipopolysaccharide (LPS)], endogenous ethanol, bile acids (BAs), and short-chain fatty acids (SCFAs). FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; TGR5, G-protein-coupled bile acid receptor; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; UDCA, ursodeoxycholic acid.
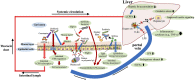
Figure 4
Roles of probiotics against NAFLD. Probiotics enhance the barrier function of the gut [e.g., mucus layer, secretory IgA (SIgA) levels and tight junction tension], and improve the gut microbiota composition, bile acid (BA) homeostasis, and short-chain fatty acids (SCFAs) production. Restored gut barrier function and gut microbiota reduce portal transport of lipopolysaccharide (LPS), and therefore decrease LPS–toll-like receptor 4(TLR4) signaling-mediated inflammatory cytokine [e.g., tumor necrosis factor-α (TNF-α)] production in the liver. Probiotics also reduce intestinal absorption of long-chain polyunsaturated fatty acid (LCPFA) by consuming intestinal LCPFA. These beneficial roles of probiotics are involved in the underlying mechanisms of protection against NAFLD. PIgR, polymeric Ig recetor.
References
Publication types
LinkOut - more resources
Full Text Sources