Pathogenesis of taste impairment and salivary dysfunction in COVID-19 patients - PubMed (original) (raw)
Review
Pathogenesis of taste impairment and salivary dysfunction in COVID-19 patients
Yasuo Okada et al. Jpn Dent Sci Rev. 2021 Nov.
Abstract
Coronavirus disease 2019 (COVID-19) is a highly transmissible pandemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The characteristics of the disease include a broad range of symptoms from mild to serious to death, with mild pneumonia to acute respiratory distress syndrome and complications in extrapulmonary organs. Taste impairment and salivary dysfunction are common early symptoms in COVID-19 patients. The mouth is a significant entry route for SARS-COV-2, similar to the nose and eyes. The cells of the oral epithelium, taste buds, and minor and major salivary glands express cell entry factors for SARS-COV-2, such as ACE2, TMPRSS2, and Furin. We describe the occurrence of taste impairment and salivary dysfunction in COVID-19 patients and show immunohistochemical findings regarding the cell entry factors in the oral tissue. We review and describe the pathogeneses of taste impairment and salivary dysfunction. Treatment for the oral disease is also described. Recently, it was reported that some people experience persistent and prolonged taste impairment and salivary dysfunction, described as post-COVID-19 syndrome or long COVID-19, after the acute illness of the infection has healed. To resolve these problems, it is important to understand the pathogenesis of oral complications. Recently, important advances have been reported in the understanding of gustatory impairment and salivary dysfunction. Although some progress has been made, considerable effort is still required for in-depth elucidation of the pathogenesis.
Keywords: COVID-19; Gustatory impairment; Long COVID; Pathogenesis; SARS-CoV-2; Salivary gland disorder; Taste impairment; Xerostomia.
© 2021 The Authors.
Figures
Fig. 1
Patients with symptoms % [[131], [132], [133]]. (A) Taste disorder. (B) Xerostomia.
Fig. 2
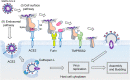
Model of the SARS-CoV-2 entry mechanism. SARS-CoV-2 utilizes two host entry routes. One is a surface membrane pathway (I), and the other route is the endosomal pathway (II). (I) In the surface membrane pathway, the RBD of the viral spike protein (S1 subunit) initially binds to the host receptor ACE2. Subsequently, Furin cleaves the spike protein at the S1/S2 site, and then the S1 subunit is dissociated from the rest of the spike protein. Another membrane-bound protease, TMPRSS2, then cleaves at the S2′ site of the S2 subunit to expose the fusion peptide for host cell membrane fusion. These events lead to a series of conformational changes that result in fusion between the viral envelope and the host cell membrane. The viral genome is released into the host cell cytoplasm. (II) Following interaction of the spike protein with the host cell receptor ACE2 on the cell membrane, the virus is endocytosed. The spike protein is processed by cathepsin L for cleavage to S1 and S2 in the endosome, which allows fusion of the viral membrane with the endosomal membrane. After that, the virus genome is released.
Fig. 3
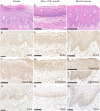
Expression of ACE2. Expression of ACE2 was observed in the nuclei and cytoplasm of the spinous and basal cell layers of the epithelium and endothelial cells of the tongue (B), oral floor (F) and buccal mucosa (J). Expression of TMPRSS2 was observed in the cell membrane of the spinous cell layers of the epithelium and endothelial cells of the tongue (C), cell membrane of the spinous cell layers and nuclei of the spinous and basal cell layers, endothelial cells of the oral floor (G), cell membrane of the spinous cell layers and nuclei of the basal cell layers, and endothelial cells of the buccal mucosa (K, L). Expression of Furin was observed in a dotted pattern and the cytoplasm of the spinous and basal cell layers of the epithelium of the tongue (D), oral floor (H) and buccal mucosa (M). Scale bars: A; 250 μm, B; 100 μm, C; 250 μm, D-H; 100 μm, I; 500 μm, J–L; 100 μm, M; 50 μm.
Fig. 4
Expression of ACE2 was observed in the serous cells, ductal epithelium and adipocytes of the parotid gland (B), serous demilunes, ductal epithelium and endothelial cells of the sublingual gland (F) and the buccal gland (J). Expression of TMPRSS2 was observed in the serous cells, ductal epithelium and adipocytes of the parotid gland (C), serous demilunes, ductal epithelium and endothelial cells of the sublingual gland (G) and the buccal gland (K). Expression of Furin was observed in the serous cells and ductal epithelium of the parotid gland (D), serous demilunes, ductal epithelium of the sublingual gland (H) and the buccal gland (L). Scale bars: A–D; 50 μm, E–L; 100 μm.
Fig. 5
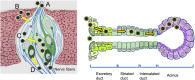
Hypothetical SARS-CoV-2 cell entry mechanism in the taste bud and salivary gland. Taste bud (left). A; Microvilli of taste sensory cells allow SARS-CoV-2 entry into the cells. B; Non-ACE2-expressing gustatory cells are infected through ACE2-positive neighboring cells. C; SARS-CoV-2 directly invades taste receptor cells via cell surface ACE2 and TMPRSS2 expression. D; SARS-CoV-2 neuroinvasion can occur at the neural-mucosal interface by transmucosal entry via regional nervous structures. (a), Type I cell; (b), Type II cell; (c), Type III cell; (d) basal cell. Salivary gland (right). SARS-CoV-2 initially enters epithelial cells close to the salivary duct orifice and/or lining salivary gland ducts through ACE2 binding. TMPRSS2 and Furin are also expressed in salivary gland ducts. Secretary cells in acinus are eventually infected with the virus.
Similar articles
- Severe acute respiratory syndrome coronavirus 2 pathology and cell tropism in tongue tissues of COVID-19 autopsies.
Ma L, Liu Q, Wang M, Liu L, Hu Z, Zhou Y, Liu J. Ma L, et al. Front Cell Infect Microbiol. 2024 Jun 21;14:1394721. doi: 10.3389/fcimb.2024.1394721. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38975331 Free PMC article. - SARS-CoV-2 Infection and Taste Alteration: An Overview.
Scotto G, Fazio V, Lo Muzio E, Lo Muzio L, Spirito F. Scotto G, et al. Life (Basel). 2022 May 6;12(5):690. doi: 10.3390/life12050690. Life (Basel). 2022. PMID: 35629357 Free PMC article. Review. - Xerostomia and Taste Alterations in COVID-19.
Belchior Fontenele MN, Pedrosa MDS. Belchior Fontenele MN, et al. Ear Nose Throat J. 2021 Apr;100(2_suppl):186S-187S. doi: 10.1177/0145561320982686. Epub 2020 Dec 23. Ear Nose Throat J. 2021. PMID: 33356523 - Existence of SARS-CoV-2 Entry Molecules in the Oral Cavity.
Sakaguchi W, Kubota N, Shimizu T, Saruta J, Fuchida S, Kawata A, Yamamoto Y, Sugimoto M, Yakeishi M, Tsukinoki K. Sakaguchi W, et al. Int J Mol Sci. 2020 Aug 20;21(17):6000. doi: 10.3390/ijms21176000. Int J Mol Sci. 2020. PMID: 32825469 Free PMC article. - COVID-19 manifestation in the oral cavity - a narrative literature review.
Kusiak A, Cichońska D, Tubaja M, Skorek A, Jereczek-Fossa BA, Corrao G, Marvaso G, Alterio D. Kusiak A, et al. Acta Otorhinolaryngol Ital. 2021 Oct;41(5):395-400. doi: 10.14639/0392-100X-N1584. Acta Otorhinolaryngol Ital. 2021. PMID: 34734574 Free PMC article. Review.
Cited by
- Severe acute respiratory syndrome coronavirus 2 pathology and cell tropism in tongue tissues of COVID-19 autopsies.
Ma L, Liu Q, Wang M, Liu L, Hu Z, Zhou Y, Liu J. Ma L, et al. Front Cell Infect Microbiol. 2024 Jun 21;14:1394721. doi: 10.3389/fcimb.2024.1394721. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38975331 Free PMC article. - Impaired metal perception and regulation of associated human foliate papillae tongue transcriptome in long-COVID-19.
Danzer B, Jukic M, Dunkel A, Andersen G, Lieder B, Schaudy E, Stadlmayr S, Lietard J, Michel T, Krautwurst D, Haller B, Knolle P, Somoza M, Lingor P, Somoza V. Danzer B, et al. Sci Rep. 2024 Jul 4;14(1):15408. doi: 10.1038/s41598-024-66079-w. Sci Rep. 2024. PMID: 38965271 Free PMC article. - Psychometric evaluation of an adult post-COVID-19 symptom tool: a development and validation study.
Kuo PY, Chen PH, Tsai SF, Lin WL, Hung CT, Huang SM. Kuo PY, et al. Sci Rep. 2024 Jan 5;14(1):664. doi: 10.1038/s41598-024-51287-1. Sci Rep. 2024. PMID: 38182859 Free PMC article. - Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies.
Zadeh FH, Wilson DR, Agrawal DK. Zadeh FH, et al. Arch Microbiol Immunol. 2023;7(2):36-61. Epub 2023 May 9. Arch Microbiol Immunol. 2023. PMID: 37388279 Free PMC article. - Treatments of COVID-19-Associated Taste and Saliva Secretory Disorders.
Tsuchiya H. Tsuchiya H. Dent J (Basel). 2023 May 25;11(6):140. doi: 10.3390/dj11060140. Dent J (Basel). 2023. PMID: 37366663 Free PMC article. Review.
References
- Centers for Disease Control and Prevention; 2021. Clinical presentation, summary of recent changes, interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) updated Feb. 16, 2021, COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-manageme... [Accessed 30 April]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. - PubMed
- Johns Hopkins University & Medicine . 2021. Coronavirus resource center, critical trends, mortality analyses.https://coronavirus.jhu.edu/data/mortality April 15.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous