The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence - PubMed (original) (raw)
Review
. 2021 Jun 23:2021:6678662.
doi: 10.1155/2021/6678662. eCollection 2021.
Affiliations
- PMID: 34257817
- PMCID: PMC8249127
- DOI: 10.1155/2021/6678662
Review
The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence
Huan Yi et al. Oxid Med Cell Longev. 2021.
Abstract
Metabolic diseases have become major public health issues worldwide. Searching for effective drugs for treating metabolic diseases from natural compounds has attracted increasing attention. Quercetin, an important natural flavonoid, is extensively present in fruits, vegetables, and medicinal plants. Due to its potentially beneficial effects on human health, quercetin has become the focus of medicinal attention. In this review, we provide a timely and comprehensive summary of the pharmacological advances and clinical data of quercetin in the treatment of three metabolic diseases, including diabetes, hyperlipidemia, and nonalcoholic fatty liver disease (NAFLD). Accumulating evidences obtained from animal experiments prove that quercetin has beneficial effects on these three diseases. It can promote insulin secretion, improve insulin resistance, lower blood lipid levels, inhibit inflammation and oxidative stress, alleviate hepatic lipid accumulation, and regulate gut microbiota disorders in animal models. However, human clinical studies on the effects of quercetin in diabetes, hyperlipidemia, and NAFLD remain scarce. More clinical trials with larger sample sizes and longer trial durations are needed to verify its true effectiveness in human subjects. Moreover, another important issue that needs to be resolved in future research is to improve the bioavailability of quercetin. This review may provide valuable information for the basic research, drug development, and clinical application of quercetin in the treatment of metabolic diseases.
Copyright © 2021 Huan Yi et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures
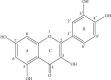
Figure 1
The chemical structure of quercetin.
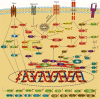
Figure 2
Quercetin can improve diabetes, hyperlipidemia, and NAFLD by modulating the marked targets. ↑ indicates increase and ↓ indicates decrease; → indicates stimulatory effect and ⊣ indicates inhibitory effect. In the upper right corner, a represents the effect of quercetin on diabetes, b represents the effect of quercetin on hyperlipidemia, and c represents the effect of quercetin on NAFLD. ABCA1: ATP-binding cassette transporter A1; Abcg5: ATP-binding cassette subfamily G member 5; Acaca: acetyl-coenzyme A carboxylase α; Akt: protein kinase B; Aldh1b1: aldehyde dehydrogenase 1 family member B1; AMPK: adenosine monophosphate-activated protein kinase; Apoa4: apolipoprotein A-IV; ATF-6_α_: activating transcriptional factor 6_α_; CAT: catalase; CD36: cluster of differentiation 36; cGMP: cyclic 3,5-guanosine monophosphate; CHOP: CCAAT/enhancer-binding protein homologous protein; CYP2E1: cytochrome P450 2E1; eNOS: endothelial nitric oxide synthase; FABP1: fatty acid-binding protein 1; FAS: fatty acid synthase; FAT/CD36: fatty acid translocase CD36; FATP5: fatty acid transport protein 5; FFAs: free fatty acids; FOXA1: forkhead box protein A1; FXR1: farnesoid X receptor 1; FAS: fatty acid synthase; FBPase: fructose-bisphosphatase; Fnta: farnesyltransferase CAAX box α; G6Pase: glucose-6-phosphate; G3PDH: glycerol-3-phosphate dehydrogenase; G6PDH: glucose-6-phosphate dehydrogenase; GCK: glucokinase; GLUT4: glucose transporter type 4; Gpam: glycerol-3-phosphate acyltransferase mitochondrial; GR: glutathione reductase; GRP78: glucose regulated protein 78; GSH: glutathione; GSH-Px: glutathione peroxidase; GST: glutathione-S-transferase; HDL: high-density lipoprotein; Hmgb1: high-mobility group box 1; HMG-CoA reductase: 3-hydroxy-3-methylglutaryl-CoA reductase; Ikk-β: inhibitor κ_B kinase β; IL-1_β: interleukin-1_β_; IL-6: interleukin-6; iNOS: inducible nitric oxide synthase; InsR: insulin receptor; IRE1_α_: inositol-requiring transmembrane kinase/endoribonuclease 1_α_; IRS-1: insulin receptor substrate-1; JNK: c-Jun-NH2 terminal kinase; LDL: low-density lipoprotein; LXR_α_: liver X receptor α; MDA: malondialdehyde; MPO: myeloperoxidase; MSR1: macrophage scavenger receptor 1; NF-κ_B: nuclear factor-κ_B; NO: nitric oxide; NPC1L1: Niemann-Pick C1-like 1; OPN: osteopontin; ox-LDL: oxidized low-density lipoprotein; P38 MAPK: P38 mitogen-activated protein kinases; PEPCK: phosphoenolpyruvate carboxylase; PERK: protein kinase-like endoplasmic reticulum kinase; PI3K: phosphatidyl inositol 3 kinase; Pon1: paraoxonase1; PPAR_α: peroxisome proliferator-activated receptor α; PPAR_γ: peroxisome proliferator-activated receptor γ; Ptgs2: cyclooxygenase-2; PTP-1B: phosphatase-1B; Ser9: phosphorylated glycogen synthase kinase 3_β_; SHP: small heterodimer partner; SIRT1: sirtuin 1; SOCS3: suppressor of cytokine signaling 3; SOD: superoxide dismutase; SREBP-1c: sterol regulatory element-binding protein-1c; TBARS: thiobarbituric acid-reactive substances; TC: total cholesterol; TG: triglyceride; TGR5: Takeda G protein-coupled receptor 5; TLR-4: Toll-like receptor-4; TNFR: tumor necrosis factor receptor; TNF-α: tumor necrosis factor-α; VEGF: vascular endothelial growth factor; VEGFR2: vascular endothelial growth factor receptor 2; VLDL: very low-density lipoprotein; XBP1s: X-box-binding protein 1.
Similar articles
- Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence.
Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, Du H, Xu T, Jiang G, Fan G. Xu X, et al. Biomed Pharmacother. 2021 Jan;133:110984. doi: 10.1016/j.biopha.2020.110984. Epub 2020 Nov 10. Biomed Pharmacother. 2021. PMID: 33186794 Review. - Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice.
Yang H, Yang T, Heng C, Zhou Y, Jiang Z, Qian X, Du L, Mao S, Yin X, Lu Q. Yang H, et al. Phytother Res. 2019 Dec;33(12):3140-3152. doi: 10.1002/ptr.6486. Epub 2019 Aug 26. Phytother Res. 2019. PMID: 31452288 - Pharmacological Activity of Flavonoid Quercetin and Its Therapeutic Potential in Testicular Injury.
Zhang X, Tang Y, Lu G, Gu J. Zhang X, et al. Nutrients. 2023 May 8;15(9):2231. doi: 10.3390/nu15092231. Nutrients. 2023. PMID: 37432408 Free PMC article. Review. - Managing metabolic diseases: The roles and therapeutic prospects of herb-derived polysaccharides.
Xu X, Wang L, Zhang K, Zhang Y, Fan G. Xu X, et al. Biomed Pharmacother. 2023 May;161:114538. doi: 10.1016/j.biopha.2023.114538. Epub 2023 Mar 15. Biomed Pharmacother. 2023. PMID: 36931026 Review. - The roles of quercetin in diabetes mellitus and related metabolic disorders; special focus on the modulation of gut microbiota: A comprehensive review.
Roshanravan N, Askari SF, Fazelian S, Ayati MH, Namazi N. Roshanravan N, et al. Crit Rev Food Sci Nutr. 2023;63(17):2990-3003. doi: 10.1080/10408398.2021.1983765. Epub 2021 Oct 7. Crit Rev Food Sci Nutr. 2023. PMID: 34620011 Review.
Cited by
- Quercetin supplementation in metabolic syndrome: nutrigenetic interactions with the Zbtb16 gene variant in rodent models.
Kábelová A, Malínská H, Marková I, Hüttl M, Liška F, Chylíková B, Šeda O. Kábelová A, et al. Genes Nutr. 2024 Oct 25;19(1):22. doi: 10.1186/s12263-024-00757-2. Genes Nutr. 2024. PMID: 39455928 Free PMC article. - Single oral administration of quercetin glycosides prevented acute hyperglycemia by promoting GLUT4 translocation in skeletal muscles through the activation of AMPK in mice.
Yamashita Y, Jiang H, Okada F, Kitakaze T, Yoshioka Y, Ashida H. Yamashita Y, et al. J Clin Biochem Nutr. 2024 Jan;74(1):37-46. doi: 10.3164/jcbn.23-30. Epub 2023 Aug 26. J Clin Biochem Nutr. 2024. PMID: 38292121 Free PMC article. - Potential Properties of Natural Nutraceuticals and Antioxidants in Age-Related Eye Disorders.
Maiuolo J, Bulotta RM, Oppedisano F, Bosco F, Scarano F, Nucera S, Guarnieri L, Ruga S, Macri R, Caminiti R, Musolino V, Gliozzi M, Carresi C, Cardamone A, Coppoletta A, Nicita M, Carnevali A, Scorcia V, Mollace V. Maiuolo J, et al. Life (Basel). 2022 Dec 27;13(1):77. doi: 10.3390/life13010077. Life (Basel). 2022. PMID: 36676026 Free PMC article. Review. - A Joint Approach of Morphological and UHPLC-HRMS Analyses to Throw Light on the Autochthonous 'Verdole' Chestnut for Nutraceutical Innovation of Its Waste.
Ferrara E, Pecoraro MT, Cice D, Piccolella S, Formato M, Esposito A, Petriccione M, Pacifico S. Ferrara E, et al. Molecules. 2022 Dec 15;27(24):8924. doi: 10.3390/molecules27248924. Molecules. 2022. PMID: 36558057 Free PMC article. - Blackberries and Mulberries: Berries with Significant Health-Promoting Properties.
Martins MS, Gonçalves AC, Alves G, Silva LR. Martins MS, et al. Int J Mol Sci. 2023 Jul 27;24(15):12024. doi: 10.3390/ijms241512024. Int J Mol Sci. 2023. PMID: 37569399 Free PMC article. Review.
References
- Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice. 2019;157 doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
- Scott R. S., Lintott C. J., Wilson M. J. Simvastatin and side effects. The New Zealand Medical Journal. 1991;104(924):493–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical