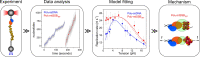
DNA replication: In vitro single-molecule manipulation data analysis and models - PubMed (original) (raw)
Review
DNA replication: In vitro single-molecule manipulation data analysis and models
Javier Jarillo et al. Comput Struct Biotechnol J. 2021.
Abstract
DNA replication is a key biochemical process of the cell cycle. In the last years, analysis of in vitro single-molecule DNA replication events has provided new information that cannot be obtained with ensembles studies. Here, we introduce crucial techniques for the proper analysis and modelling of DNA replication in vitro single-molecule manipulation data. Specifically, we review some of the main methods to analyze and model the real-time kinetics of the two main molecular motors of the replisome: DNA polymerase and DNA helicase. Our goal is to facilitate access to and understanding of these techniques to promotetheir use in the study of DNA replication at the single-molecule level. A proper analysis of single-molecule data is crucial to obtain a detailed picture of, among others, the kinetics rates, equilibrium contants and conformational changes of the system under study. The techniques presented here have been used or can be adapted to study the operation of other proteins involved in nucleic acids metabolism.
Keywords: DNA polymerase; DNA replication; DNA unwinding; Helicase; Real-time kinetics; Single-molecule.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Graphical abstract
Fig. 1
Simplified view of the core components of the mitochondrial DNA replisome. Helicase opens the DNA fork separating the two strands. The leading strand, is replicated directly by the DNA polymerase, and the lagging strand, is initially bound by SSB proteins for later replication. In other replisomes, several primase subunits usually associate with the helicase to prime replication of the lagging strand, which is replicated in the opposite direction in the form of short Okazaki fragment (not shown).
Fig. 2
A) Example of a primer extension (p.e.) DNA replication experiment with optical tweezers. The two ends of a dsDNA molecule containing a ssDNA gap in the middle are attached between two micron-sized beads: one (grey sphere) held by the optical trap (highly focalized laser, red) and the other (blue sphere) held by suction on top of a micropipette. As replication proceeds, ssDNA is converted into the more rigid dsDNA changing the distance between the beads. The change in distance is registered and later processed to obtain the polymerase trajectory (template position versus time). B) Representative replication traces showing transient pause events (red) intercalated with active replication events (black). The inset shows the velocity histogram. C) Pause length frequency distributions. Depending on the experimental conditions, the pause length frequency distribution can be compatible with a single (red line) or a double exponential distribution (green line). Other distribuitions are also possible. D) Diagram of a magnetic tweezers experiment measuring the DNA unwinding activity of a single replicative helicase. One of the ends of the dsDNA (bearing a helicase loading site) is attached to a glass surface and the other end to a super-paramagnetic microsphere manipulated by the magnetic field. E) Representative unwinding trace showing the binning in displacement and illustrating the determination of first passage times τi. F) First-passage time (FPT) distribution. Experimental data (blue circles) are fitted by a model with pauses and forward and backward stepping (blue line). Predicted FPT distribution without pauses (orange) and with only forward stepping (green) are shown for comparison. G) Experimental configuration to measure the replication of ssDNA covered with SSB with optical tweezers. When the replication velocity is slow, direct identification of pauses and maximum replication velocity V is not possible, neither from the traces nor from the velocity histogram. H) Identification of peak velocities Vpeak with prominence greater than P (which avoids the selection of secondary peaks). Traces are averaged on a time window τ then the instantaneous velocity is represented versus time to proceed to peak velocity identification. I) The mean of the velocity peaks Vpeak,mean is computed for each prominence P for different time windows τ. For an intermediate value of the prominence P a clear plateau is present in the Vpeak,mean(τ) plot, whose value gives the maximum velocity V. (Panel A in this Figure is adapted from Ref. ; Panels B and C are from Ref. ; Panels D, E and F are from Ref. ; Panels G, H and I are adapted from Ref. . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
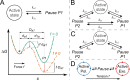
Fig. 3
Kinetic models for pause and active states of DNA polymerases. Parameters kij denotes the transition rate from state i to state j. A) Top: Model with a unique pause state. Bottom: Schematic representation of the effect of an external force f on the free energy landscape projected along the displacement coordinate in the direction of the force. The free energy reduction is given by the work done by the force: f·da1 for the activation state, f·Da1 for the final state. B) Cyclic model with two pause states. In this model direct transitions between pause states are allowed. C) Linear model with two different pauses states. In this model it is not possible to go directly from one pause state to the other one, without passing through the active state. D) Model of polymerization-exonucleolysis transitions mediated by a pause state. DNA tension induces entrance into exonucleolysis through the pause intermediate , . (Panels A, B and C adapted from Refs. [69], [73]).
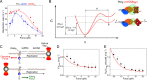
Fig. 4
Comparison between the effects of mechanical tension on the DNA (A and B) and mechanical load applied to the DNA polymerase (C-E). A) Effect of mechanical tension of the primer extension replication rate of the mitochondrial DNA polymerase in the absence (blue) and presence of the mitochondrial SSB (mtSSBWT). Dots represent experimental data, and lines the best fitted theoretical models. Eq. (8) for ssDNA and Eq. (11) for SSB covered ssDNA. B) Comparison of polymerase-SSB coupling behavior at different tensions (f). The energy landscapes (left) between the coupled state A and the uncoupled state B, for low force (solid line) and for medium force (dashed line), show how force destabilizes the polymerase-SSB coupling. The diagrams (right) represent the polymerase-SSB coupling reduction due to force. [This decoupling effect is modeled by Eq. (10).] C) Diagram of a primer extension experiment applying opposing (top) or aiding (bottom) force on a DNA polymerase. D) Effect of load on the maximum replication rate Vmax at saturating dNTP. E) Ratio of the apparent nucleotide constant and the maximum replication rate, KM/Vmax, (Michaelis-Menten parameters of the reaction) as a function of the force acting on the polymerase. (Panels A and B from Ref. , Panels C, D and E from [70]). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5
A) Diagram of a strand displacement DNA replication experiment with optical tweezers. (Left) The two ends of a DNA hairpin are attached between two micron-sized beads (grey and blue spheres) one held by the optical trap and the other held by suction on top of a micropipette. Double parallel lines represent double stranded DNA (dsDNA). (Right) During strand displacement conditions (s.d.), the DNA polymerase (purple triangle) opens the DNA fork, replicates one strand (blue line), and displaces the other (red line). B) Scheme for the polymerase (purple triangle) dynamics during strand displacement DNA synthesis. L denotes the length in nucleotides of the DNA template, l the number of nucleotides replicated, m the number of base pairs opened between the polymerase and the DNA fork, while M stands from the number of base pairs that are destabilized by the polymerase (purple circle). All variables used in the strand displacement replication model are described in Section 3.3. (Adapted from .) C) DNA polymerases with high fork destabilization energies, ΔGd, would present s.d. rates Vsd similar to those found during primer extension Vpe (red line). On the contrary, DNA polymerases with low ΔGd present lower Vsd/Vpe ratios with stronger force dependencies (green dashed dotted line). (For all lines M=1.) D) Variation of the force dependent Vsd/Vpe ratio with the interaction range M. Higher M values yield stronger force dependencies. Different values of M can fit the same set of data with different interaction intensities ΔGd. (Values of the lines in this panel are: ΔGd=2.8kBT for M=1, ΔGd=2.0kBT for M=2,ΔGd=1.6kBT for M=4,ΔGd=1.5kBT for M=8.) E) Helicases are classified as active or passive according to their ability to destabilize the fork, parameterized by the interaction intensity ΔGd. They are optimally active when ΔGd is of the order of the higher base pair binding energy ΔGGC. The coordinate operation of a polymerase and a helicase can increase the effective interaction intensity ΔGd. F) Helicase with steps δ larger than one require the simultaneous opening of δ base pairs, implying a stronger tension dependence. (Active ΔGd=1.2kBT, M=6; passive ΔGd=0.) (Panels E and F are from .) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Similar articles
- In vitro single-molecule manipulation studies of viral DNA replication.
Bocanegra R, Plaza G A I, Ibarra B. Bocanegra R, et al. Enzymes. 2021;49:115-148. doi: 10.1016/bs.enz.2021.09.001. Epub 2021 Oct 13. Enzymes. 2021. PMID: 34696830 - Observing protein dynamics during DNA-lesion bypass by the replisome.
Wilkinson EM, Spenkelink LM, van Oijen AM. Wilkinson EM, et al. Front Mol Biosci. 2022 Sep 21;9:968424. doi: 10.3389/fmolb.2022.968424. eCollection 2022. Front Mol Biosci. 2022. PMID: 36213113 Free PMC article. Review. - Single-Molecule Optical-Trapping Techniques to Study Molecular Mechanisms of a Replisome.
Sun B, Wang MD. Sun B, et al. Methods Enzymol. 2017;582:55-84. doi: 10.1016/bs.mie.2016.08.001. Epub 2016 Oct 27. Methods Enzymol. 2017. PMID: 28062045 - Single-molecule perspectives on helicase mechanisms and functions.
Sun B, Wang MD. Sun B, et al. Crit Rev Biochem Mol Biol. 2016;51(1):15-25. doi: 10.3109/10409238.2015.1102195. Epub 2015 Nov 5. Crit Rev Biochem Mol Biol. 2016. PMID: 26540349 Review. - DNA replication machinery: Insights from in vitro single-molecule approaches.
Bocanegra R, Ismael Plaza GA, Pulido CR, Ibarra B. Bocanegra R, et al. Comput Struct Biotechnol J. 2021 Apr 20;19:2057-2069. doi: 10.1016/j.csbj.2021.04.013. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33995902 Free PMC article. Review.
Cited by
- Cooperative kinetics of ligand binding to linear polymers.
Villaluenga JPG, Cao-García FJ. Villaluenga JPG, et al. Comput Struct Biotechnol J. 2022 Jan 6;20:521-533. doi: 10.1016/j.csbj.2021.12.043. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35495112 Free PMC article.
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. - DOI
Publication types
LinkOut - more resources
Full Text Sources