Preventing Thrombohemorrhagic Complications of Heparinized COVID-19 Patients Using Adjunctive Thromboelastography: A Retrospective Study - PubMed (original) (raw)
. 2021 Jul 14;10(14):3097.
doi: 10.3390/jcm10143097.
Anthony V Thomas 1, John E Stillson 1, Laura Gillespie 2, Rashid Z Khan 3, Nuha Zackariya 1, Faadil Shariff 4, Mahmoud Al-Fadhl 5, Nicolas Mjaess 5, Peter D Miller 6, Michael T McCurdy 7, Daniel H Fulkerson 8, Joseph B Miller 9, Hau C Kwaan 10, Ernest E Moore 11, Hunter B Moore 11, Matthew D Neal 12, Peter L Martin 13, Mark L Kricheff 14, Mark M Walsh 1 5 14
Affiliations
- PMID: 34300263
- PMCID: PMC8303660
- DOI: 10.3390/jcm10143097
Preventing Thrombohemorrhagic Complications of Heparinized COVID-19 Patients Using Adjunctive Thromboelastography: A Retrospective Study
Connor M Bunch et al. J Clin Med. 2021.
Abstract
Background: The treatment of COVID-19 patients with heparin is not always effective in preventing thrombotic complications, but can also be associated with bleeding complications, suggesting a balanced approach to anticoagulation is needed. A prior pilot study supported that thromboelastography and conventional coagulation tests could predict hemorrhage in COVID-19 in patients treated with unfractionated heparin or enoxaparin, but did not evaluate the risk of thrombosis.
Methods: This single-center, retrospective study included 79 severely ill COVID-19 patients anticoagulated with intermediate or therapeutic dose unfractionated heparin. Two stepwise logistic regression models were performed with bleeding or thrombosis as the dependent variable, and thromboelastography parameters and conventional coagulation tests as the independent variables.
Results: Among all 79 patients, 12 (15.2%) had bleeding events, and 20 (25.3%) had thrombosis. Multivariate logistic regression analysis identified a prediction model for bleeding (adjusted R2 = 0.787, p < 0.001) comprised of increased reaction time (p = 0.016), decreased fibrinogen (p = 0.006), decreased D-dimer (p = 0.063), and increased activated partial thromboplastin time (p = 0.084). Multivariate analysis of thrombosis identified a weak prediction model (adjusted R2 = 0.348, p < 0.001) comprised of increased D-dimer (p < 0.001), decreased reaction time (p = 0.002), increased maximum amplitude (p < 0.001), and decreased alpha angle (p = 0.014). Adjunctive thromboelastography decreased the use of packed red cells (p = 0.031) and fresh frozen plasma (p < 0.001).
Conclusions: Significantly, this study demonstrates the need for a precision-based titration strategy of anticoagulation for hospitalized COVID-19 patients. Since severely ill COVID-19 patients may switch between thrombotic or hemorrhagic phenotypes or express both simultaneously, institutions may reduce these complications by developing their own titration strategy using daily conventional coagulation tests with adjunctive thromboelastography.
Keywords: COVID-19; anticoagulants; blood coagulation; blood coagulation tests; coagulopathy; hemorrhage; heparin; thromboelastography; thrombosis.
Conflict of interest statement
E.E.M., H.B.M., M.D.N., and M.M.W. have received research grants from Haemonetics Inc. (Boston, MA, USA) outside the submitted work. M.M.W. has received honoraria from Alexion Pharmaceuticals (Boston, MA, USA). M.D.N. has received an honorarium from Haemonetics for a speaking engagement and research support from Janssen Pharmaceuticals (Beerse, Belgium) and Noveome (Pittsburgh, PA, USA) outside the submitted work. He has served as a consultant to Janssen and CSL Behring (King of Prussia, PA, USA), and serves on the Scientific Advisory Board of Haima Therapeutics (Cleveland, OH, USA).
Figures
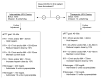
Figure 1
Sample TEG/CCT-based protocol for UFH dosing of hospitalized COVID-19 patients. The presence/absence of a thrombosis determines initial UFH dosing. Further goal-directed titration of UFH was achieved via monitoring of aPTT and TEG parameters R and MA. Adopted from protocols for guiding heparin in ECMO, liver transplantation, cardiac surgery, and trauma surgery [22,36]. aPTT, activated partial thromboplastin time; CCT, conventional coagulation test; COVID-19, coronavirus disease 2019; ICU, intensive care unit; MA, maximum amplitude; R, reaction time; TEG, thromboelastography; UFH, unfractionated heparin.
Similar articles
- Integral Use of Thromboelastography With Platelet Mapping to Guide Appropriate Treatment, Avoid Complications, and Improve Survival of Patients With Coronavirus Disease 2019-Related Coagulopathy.
Hranjec T, Estreicher M, Rogers B, Kohler L, Solomon R, Hennessy S, Cibulas M, Hurst D, Hegazy M, Lee J, Perez D, Doctor N, Kiffin C, Pigneri D, LaGuardia H, Shaw K, Arenas J, Rosenthal A, Katz RS, Sawyer RG, Pepe PE. Hranjec T, et al. Crit Care Explor. 2020 Dec 21;2(12):e0287. doi: 10.1097/CCE.0000000000000287. eCollection 2020 Dec. Crit Care Explor. 2020. PMID: 33381763 Free PMC article. - Stratification of Bleeding Risk Using Thromboelastography in Children on Extracorporeal Membrane Oxygenation Support.
Sleeper LA, Mulone M, Diallo F, Nasr VG, Thiagarajan RR, Emani S, Emani S. Sleeper LA, et al. Pediatr Crit Care Med. 2021 Mar 1;22(3):241-250. doi: 10.1097/PCC.0000000000002657. Pediatr Crit Care Med. 2021. PMID: 33512982 - Major bleeding complications in critically ill patients with COVID-19 pneumonia.
Godier A, Clausse D, Meslin S, Bazine M, Lang E, Huche F, Cholley B, Hamada SR. Godier A, et al. J Thromb Thrombolysis. 2021 Jul;52(1):18-21. doi: 10.1007/s11239-021-02403-9. Epub 2021 Mar 1. J Thromb Thrombolysis. 2021. PMID: 33646501 Free PMC article. - AGA Clinical Practice Update: Coagulation in Cirrhosis.
O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. O'Leary JG, et al. Gastroenterology. 2019 Jul;157(1):34-43.e1. doi: 10.1053/j.gastro.2019.03.070. Epub 2019 Apr 12. Gastroenterology. 2019. PMID: 30986390 Review. - The Role of TEG Analysis in Patients with COVID-19-Associated Coagulopathy: A Systematic Review.
Hartmann J, Ergang A, Mason D, Dias JD. Hartmann J, et al. Diagnostics (Basel). 2021 Jan 26;11(2):172. doi: 10.3390/diagnostics11020172. Diagnostics (Basel). 2021. PMID: 33530346 Free PMC article. Review.
Cited by
- A retrospective multicenter cohort study of the association between anti-Factor Xa values and death, thromboembolism, and bleeding in patients with critical COVID-19.
Jonmarker S, Litorell J, Alarcon F, Al-Abani K, Björkman S, Farm M, Grip J, Söderberg M, Hollenberg J, Wahlin RR, Kander T, Rimling L, Mårtensson J, Joelsson-Alm E, Dahlberg M, Cronhjort M. Jonmarker S, et al. Thromb J. 2023 Oct 2;21(1):101. doi: 10.1186/s12959-023-00541-z. Thromb J. 2023. PMID: 37784131 Free PMC article. - Reply to Bareille et al. Are Viscoelastometric Assays of Old Generation Ready for Disposal? Comment on "Volod et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860".
Volod O, Bunch CM, Miller J, Moore EE, Moore HB, Kwaan HC, Patel SS, Wiarda G, Aboukhaled M, Thomas SG, Fulkerson D, Erdman L, Tincher A, Walsh MM. Volod O, et al. J Clin Med. 2023 Jan 6;12(2):478. doi: 10.3390/jcm12020478. J Clin Med. 2023. PMID: 36675408 Free PMC article. - Serial Thromboelastography and the Development of Venous Thromboembolism in Critically Ill Patients With COVID-19.
Marvi TK, Stubblefield WB, Tillman BF, Tenforde MW, Patel MM, Lindsell CJ, Self WH, Grijalva CG, Rice TW. Marvi TK, et al. Crit Care Explor. 2022 Jan 18;4(1):e0618. doi: 10.1097/CCE.0000000000000618. eCollection 2022 Jan. Crit Care Explor. 2022. PMID: 35072082 Free PMC article. - Viscoelastic Hemostatic Assays for Postpartum Hemorrhage.
Liew-Spilger AE, Sorg NR, Brenner TJ, Langford JH, Berquist M, Mark NM, Moore SH, Mark J, Baumgartner S, Abernathy MP. Liew-Spilger AE, et al. J Clin Med. 2021 Aug 31;10(17):3946. doi: 10.3390/jcm10173946. J Clin Med. 2021. PMID: 34501395 Free PMC article. Review. - Comparison of the New Viscoelastic Coagulation Analyzer ClotPro® With ROTEM® Delta and Conventional Coagulation Tests in Critically Ill Patients With COVID-19.
Infanger L, Dibiasi C, Schaden E, Ulbing S, Wiegele M, Lacom C, Gratz J. Infanger L, et al. Front Med (Lausanne). 2021 Nov 16;8:777145. doi: 10.3389/fmed.2021.777145. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34869496 Free PMC article.
References
- Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E., Arvind V., Bose S., Charney A.W., Chen M.D. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources