Leonurine Ameliorates Oxidative Stress and Insufficient Angiogenesis by Regulating the PI3K/Akt-eNOS Signaling Pathway in H2O2-Induced HUVECs - PubMed (original) (raw)
Leonurine Ameliorates Oxidative Stress and Insufficient Angiogenesis by Regulating the PI3K/Akt-eNOS Signaling Pathway in H2O2-Induced HUVECs
Li Liao et al. Oxid Med Cell Longev. 2021.
Abstract
Thrombus is considered to be the pathological source of morbidity and mortality of cardiovascular disease and thrombotic complications, while oxidative stress is regarded as an important factor in vascular endothelial injury and thrombus formation. Therefore, antioxidative stress and maintaining the normal function of vascular endothelial cells are greatly significant in regulating vascular tension and maintaining a nonthrombotic environment. Leonurine (LEO) is a unique alkaloid isolated from Leonurus japonicus Houtt (a traditional Chinese medicine (TCM)), which has shown a good effect on promoting blood circulation and removing blood stasis. In this study, we explored the protective effect and action mechanism of LEO on human umbilical vein endothelial cells (HUVECs) after damage by hydrogen peroxide (H2O2). The protective effects of LEO on H2O2-induced HUVECs were determined by measuring the cell viability, cell migration, tube formation, and oxidative biomarkers. The underlying mechanism of antioxidation of LEO was investigated by RT-qPCR and western blotting. Our results showed that LEO treatment promoted cell viability; remarkably downregulated the intracellular generation of reactive oxygen species (ROS), malondialdehyde (MDA) production, and lactate dehydrogenase (LDH); and upregulated the nitric oxide (NO) and superoxide dismutase (SOD) activity in H2O2-induced HUVECs. At the same time, LEO treatment significantly promoted the phosphorylation level of angiogenic protein PI3K, Akt, and eNOS and the expression level of survival factor Bcl2 and decreased the expression level of death factor Bax and caspase3. In conclusion, our findings suggested that LEO can ameliorate the oxidative stress damage and insufficient angiogenesis of HUVECs induced by H2O2 through activating the PI3K/Akt-eNOS signaling pathway.
Copyright © 2021 Li Liao et al.
Conflict of interest statement
The authors have declared no other competing interests.
Figures
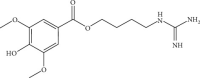
Figure 1
Chemical structure of LEO.
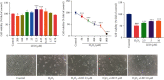
Figure 2
Detection of cell survival rate by MTT assay: (a) effect of LEO on the proliferation of HUVEC; (b) effect of H2O2 on the viability of HUVEC; (c) the survival rate of H2O2-induced injury following treatment with LEO at different concentrations; (d) effect of LEO on cell morphology after H2O2-induced injury in HUVECs. Values are presented as means ± S.D. (n = 6). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group.
Figure 3
Detection of cell migration rate and wound closure. Values are presented as means ± S.D. (n = 3). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group. The bar chart shows quantitative data for HUVEC migration with the treatment of different concentrations of LEO.
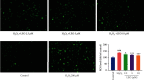
Figure 4
Evaluation for the tube formation in HUVECs. Images for the in vitro formed tubes in HUVECs. Values are presented as means ± S.D. (n = 3). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group. The bar chart shows quantitative data for HUVEC tube formation with the treatment of different concentrations of LEO.
Figure 5
LEO inhibits oxidative damages in H2O2 stimulated in HUVECs. ROS generation in HUVECs was determined by measuring DCFH fluorescence. The ROS fluorescence intensity index was presented as the percentage of the control group. Data are represented as the mean ± S.D. of three separate experiments (n = 6). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group.
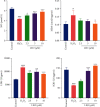
Figure 6
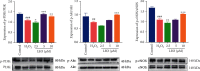
Effect of LEO on NO, MDA, LDH, and SOD levels in HUVECs treated with H2O2. Values are presented as means ± S.D. (n = 3). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group.
Figure 7
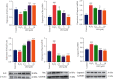
Effect of LEO on the mRNA expression and protein expression of Bax, Bcl2, and caspase3 in HUVECs: (a) effect of LEO on the mRNA expression of Bax, Bcl2, and caspase3 in HUVECs; (b) effect of LEO on the protein expression of Bax, Bcl2, and caspase3 in HUVECs. Values are presented as means ± S.D. (n = 3). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group.
Figure 8
Effect of LEO on protein expression of PI3K, p-PI3K, Akt, p-Akt, eNOS, and p-eNOS in HUVEC. Values are presented as means ± S.D. (n = 3). #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. control group; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. H2O2 group.
Figure 9
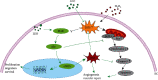
H2O2 can induce the expression of apoptotic protein Bax and inhibit the phosphorylation of PI3K/Akt by increasing the content of ROS, while LEO can promote the phosphorylation of PI3K/Akt and further promote the expression of eNOS, thus promoting the survival, proliferation, migration, and NO release of endothelial cells. At the same time, phosphorylated-Akt can also inhibit the expression of apoptotic proteins such as Bcl2 and Bax, thus inhibiting endothelial cell apoptosis induced by ROS. “←” indicates activation, and “⟝” indicates inhibition.
Similar articles
- The protective effects of a novel synthetic β-elemene derivative on human umbilical vein endothelial cells against oxidative stress-induced injury: Involvement of antioxidation and PI3k/Akt/eNOS/NO signaling pathways.
Ahmad KA, Ze H, Chen J, Khan FU, Xuezhuo C, Xu J, Qilong D. Ahmad KA, et al. Biomed Pharmacother. 2018 Oct;106:1734-1741. doi: 10.1016/j.biopha.2018.07.107. Epub 2018 Jul 30. Biomed Pharmacother. 2018. PMID: 30119249 - Tectorigenin protect HUVECs from H2O2-induced oxidative stress injury by regulating PI3K/Akt pathway.
Chen X, Zhang W, Sun L, Lian Y. Chen X, et al. Tissue Cell. 2021 Feb;68:101475. doi: 10.1016/j.tice.2020.101475. Epub 2020 Dec 29. Tissue Cell. 2021. PMID: 33385639 - Hydrogen Sulfide Protects Against High Glucose-Induced Human Umbilical Vein Endothelial Cell Injury Through Activating PI3K/Akt/eNOS Pathway.
Lin F, Yang Y, Wei S, Huang X, Peng Z, Ke X, Zeng Z, Song Y. Lin F, et al. Drug Des Devel Ther. 2020 Feb 14;14:621-633. doi: 10.2147/DDDT.S242521. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32103904 Free PMC article. - Leonurine, a potential drug for the treatment of cardiovascular system and central nervous system diseases.
Huang L, Xu DQ, Chen YY, Yue SJ, Tang YP. Huang L, et al. Brain Behav. 2021 Feb;11(2):e01995. doi: 10.1002/brb3.1995. Epub 2020 Dec 10. Brain Behav. 2021. PMID: 33300684 Free PMC article. Review. - A literature review: mechanisms of antitumor pharmacological action of leonurine alkaloid.
Cao Q, Wang Q, Wu X, Zhang Q, Huang J, Chen Y, You Y, Qiang Y, Huang X, Qin R, Cao G. Cao Q, et al. Front Pharmacol. 2023 Sep 25;14:1272546. doi: 10.3389/fphar.2023.1272546. eCollection 2023. Front Pharmacol. 2023. PMID: 37818195 Free PMC article. Review.
Cited by
- Amygdalin attenuates PM2.5-induced human umbilical vein endothelial cell injury via the TLR4/NF-κB and Bcl-2/Bax signaling pathways.
Wang B, Sun T, Sun L, Li L, Wan H, Ding Z, Ye X. Wang B, et al. Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(10):1476-1485. doi: 10.3724/abbs.2022136. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36178164 Free PMC article. - Citronellal Attenuates Oxidative Stress-Induced Mitochondrial Damage through TRPM2/NHE1 Pathway and Effectively Inhibits Endothelial Dysfunction in Type 2 Diabetes Mellitus.
Yin YL, Wang HH, Gui ZC, Mi S, Guo S, Wang Y, Wang QQ, Yue RZ, Lin LB, Fan JX, Zhang X, Mao BY, Liu TH, Wan GR, Zhan HQ, Zhu ML, Jiang LH, Li P. Yin YL, et al. Antioxidants (Basel). 2022 Nov 14;11(11):2241. doi: 10.3390/antiox11112241. Antioxidants (Basel). 2022. PMID: 36421426 Free PMC article. - Assessment of the Effect of Leonurine Hydrochloride in a Mouse Model of PCOS by Gene Expression Profiling.
Wang M, Yang L, Sun G, Shao Y, Liu Y, Yang H, Wang Y, Zhang M, Shang Y, Gu X. Wang M, et al. Genes (Basel). 2024 Apr 18;15(4):507. doi: 10.3390/genes15040507. Genes (Basel). 2024. PMID: 38674441 Free PMC article. - Fucoidan promotes angiogenesis and accelerates wound healing through AKT/Nrf2/HIF-1α signalling pathway.
Wen W, Yang L, Wang X, Zhang H, Wu F, Xu K, Chen S, Liao Z. Wen W, et al. Int Wound J. 2023 Nov;20(9):3606-3618. doi: 10.1111/iwj.14239. Epub 2023 May 18. Int Wound J. 2023. PMID: 37203309 Free PMC article. - Identification of the Antithrombotic Mechanism of Leonurine in Adrenalin Hydrochloride-Induced Thrombosis in Zebrafish via Regulating Oxidative Stress and Coagulation Cascade.
Liao L, Zhou M, Wang J, Xue X, Deng Y, Zhao X, Peng C, Li Y. Liao L, et al. Front Pharmacol. 2021 Nov 4;12:742954. doi: 10.3389/fphar.2021.742954. eCollection 2021. Front Pharmacol. 2021. PMID: 34803688 Free PMC article.
References
- Pang X.-X., Wang X. Research progress in tthrombosis and its mechanism. Medical Recapitulate. 2011;17(11):p. 1.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials