Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin - PubMed (original) (raw)
Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin
Etheresia Pretorius et al. Cardiovasc Diabetol. 2021.
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2)-induced infection, the cause of coronavirus disease 2019 (COVID-19), is characterized by acute clinical pathologies, including various coagulopathies that may be accompanied by hypercoagulation and platelet hyperactivation. Recently, a new COVID-19 phenotype has been noted in patients after they have ostensibly recovered from acute COVID-19 symptoms. This new syndrome is commonly termed Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Here we refer to it as Long COVID/PASC. Lingering symptoms persist for as much as 6 months (or longer) after acute infection, where COVID-19 survivors complain of recurring fatigue or muscle weakness, being out of breath, sleep difficulties, and anxiety or depression. Given that blood clots can block microcapillaries and thereby inhibit oxygen exchange, we here investigate if the lingering symptoms that individuals with Long COVID/PASC manifest might be due to the presence of persistent circulating plasma microclots that are resistant to fibrinolysis.
Methods: We use techniques including proteomics and fluorescence microscopy to study plasma samples from healthy individuals, individuals with Type 2 Diabetes Mellitus (T2DM), with acute COVID-19, and those with Long COVID/PASC symptoms.
Results: We show that plasma samples from Long COVID/PASC still contain large anomalous (amyloid) deposits (microclots). We also show that these microclots in both acute COVID-19 and Long COVID/PASC plasma samples are resistant to fibrinolysis (compared to plasma from controls and T2DM), even after trypsinisation. After a second trypsinization, the persistent pellet deposits (microclots) were solubilized. We detected various inflammatory molecules that are substantially increased in both the supernatant and trapped in the solubilized pellet deposits of acute COVID-19 and Long COVID/PASC, versus the equivalent volume of fully digested fluid of the control samples and T2DM. Of particular interest was a substantial increase in α(2)-antiplasmin (α2AP), various fibrinogen chains, as well as Serum Amyloid A (SAA) that were trapped in the solubilized fibrinolytic-resistant pellet deposits.
Conclusions: Clotting pathologies in both acute COVID-19 infection and in Long COVID/PASC might benefit from following a regime of continued anticlotting therapy to support the fibrinolytic system function.
Keywords: Antiplasmin; COVID-19; Fibrin(ogen); Long COVID/PASC; Microclots; Proteomics; Serum Amyloid A.
© 2021. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Fig. 1
Two trypsin digestion protocols, followed by fluorescence microscopy and proteomics of platelet poor plasma (PPP) from healthy individuals, patients with Type 2 Diabetes Mellitus (T2DM), COVID-19 and Long COVID/PASC. (1) Citrated blood was centrifuged to obtain PPP. (2) PPP were treated with trypsin to allow plasma protein digestion. Health PPP and T2DM PPP were fully degraded. COVID-19 and Long COVID/PASC sample formed a undigested pellet deposit at the bottom of the tubes. (3 and 4) For fluorescence microscopy, the supernatants were removed and the remaining 10 µL of supernatant and/or pellet samples were exposed to thioflavin T (ThT) and viewed with fluorescence microscope. Before liquid chromatography-mass spectrometry (LC–MS) based proteomics, supernatants were passed through a C18 solid phase extraction (SPE) device. (5) A second trypsin digestion protocol was followed to (6) degrade the pellet deposit in the COVID-19 and Long COVID/PASC samples. The same method was followed with healthy and T2DM PPP (although these samples did not contain a visible pellet deposit). (7) Double-trypsinized samples from controls, COVID-19 and Long COVID/PASC samples were then studied using proteomics. (Figure created with BioRender.com)
Fig. 2
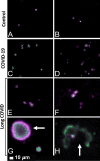
Fluorescence microscopy of haematocrit samples, showing representative micrographs of platelets. A, B show representative micrographs from healthy individuals. C, D show hyperactivated platelets in COVID-19 and E–H show representative micrographs from platelets from Long COVID/PASC samples. White arrows show aggregated platelets. Platelets in the haematocrit were incubated with the fluorescent markers PAC-1 (green fluorescence) and CD62P-PE (purple fluorescence)
Fig. 3
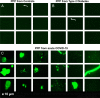
Micrographs of platelet poor plasma (PPP) (BEFORE trypsin digestion) with added thioflavin T (ThT). The marker thioflavin T (ThT) binds to anomalous (amyloid) microclots in the PPP. A Microclots in a healthy volunteer, before acute COVID-19 infection and B the same individual during Long COVID/PASC. C Representative micrographs of other patients with Long COVID/PASC
Fig. 4
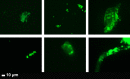
Digested supernatant of platelet poor plasma (PPP) (AFTER trypsin digestion). The marker thioflavin T (ThT) binds to anomalous (amyloid) microclots in the PPP. A Micrographs of PPP from healthy individuals; B Micrographs of PPP from Type 2 Diabetes Mellitus (T2DM). White arrows point out small areas where ThT bound in samples from controls and T2DM. C Significant microclots are visible in plasma from patients with COVID-19 as seen in the green signal in the micrographs of PPP from COVID-19
Fig. 5
Representative micrographs of Long COVID/PASC patients. Digested supernatant (PPP) after first trypsin digestion step, where supernatant was removed and thioflavin T (ThT) added to the remaining 10 µL. The marker thioflavin T (ThT) binds to anomalous microclots in the PPP
Fig. 6
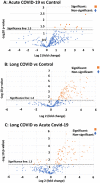
Volcano plots of the protein distribution between pairwise sample comparisons (controls vs COVID-19; controls vs Long COVID/PASC; COVID-19 vs Long COVID/PASC). Oranges dots show proteins above the significance levels as indicated by the dotted line. Foldchange is presented on the X-axis (negative values: down-regulation and positive values: upregulation). The Y-axis represents the Log-(minus) 10 of the p-values
Fig. 7
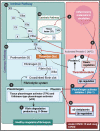
Simplified coagulation diagram (adapted from [37, 71, 72] depicting healthy and pathological processes. (1A) The intrinsic and (1B) extrinsic pathways converge into the (1C) common pathway. These pathways lead to the conversion of soluble fibrinogen to insoluble fibrin, catalysed by thrombin. (2) Tissue plasminogen activator (tPA) or urokinase-type plasminogen activator (uPA) converts plasminogen into plasmin. A healthy fibrinolytic system regulates the coagulation pathway and assists with successful lysis of the insoluble fibrin clot. (3) Plasmin cleaves fibrin into fibrin degradation products (FDPs), including D-dimer. (4) Protein C and thrombomodulin both regulate coagulation: thrombin binds to its receptor, thrombomodulin, resulting in activated protein C (APC). APC then inhibits both Va and VIIIa. (5) Dysregulated inflammatory molecules may interfere with tissue factor (TF) expression. (6) Dysregulated inflammatory molecules may also down-regulate thrombomodulin, resulting in hypercoagulation, as Va and VIIIa activities are then not sufficiently modulated. (7) Dysregulated inflammatory molecules in circulation can inhibit of the fibrinolytic system via up-regulation of plasminogen activator inhibitor-1 (PAI-1). PAI-I upregulation interferes with tissue plasminogen activator (TPA) function, and ultimately results in a dysregulated coagulation system. (8) α2-antiplasmin (α2AP) inhibits plasmin and ultimately will prevent sufficient fibrinolysis to happen. (Figure created with Biorender.com)
Similar articles
- Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC).
Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB. Pretorius E, et al. Cardiovasc Diabetol. 2022 Aug 6;21(1):148. doi: 10.1186/s12933-022-01579-5. Cardiovasc Diabetol. 2022. PMID: 35933347 Free PMC article. - Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system.
Kruger A, Vlok M, Turner S, Venter C, Laubscher GJ, Kell DB, Pretorius E. Kruger A, et al. Cardiovasc Diabetol. 2022 Sep 21;21(1):190. doi: 10.1186/s12933-022-01623-4. Cardiovasc Diabetol. 2022. PMID: 36131342 Free PMC article. - A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications.
Kell DB, Laubscher GJ, Pretorius E. Kell DB, et al. Biochem J. 2022 Feb 17;479(4):537-559. doi: 10.1042/BCJ20220016. Biochem J. 2022. PMID: 35195253 Free PMC article. Review. - Plasma proteomics show altered inflammatory and mitochondrial proteins in patients with neurologic symptoms of post-acute sequelae of SARS-CoV-2 infection.
Hanson BA, Visvabharathy L, Orban ZS, Jimenez M, Batra A, Liotta EM, DeLisle RK, Klausner JD, Cohen P, Padhye AS, Tachas G, Koralnik IJ. Hanson BA, et al. Brain Behav Immun. 2023 Nov;114:462-474. doi: 10.1016/j.bbi.2023.08.022. Epub 2023 Sep 11. Brain Behav Immun. 2023. PMID: 37704012 Free PMC article. - Neurological post-acute sequelae of SARS-CoV-2 infection.
Takao M, Ohira M. Takao M, et al. Psychiatry Clin Neurosci. 2023 Feb;77(2):72-83. doi: 10.1111/pcn.13481. Epub 2022 Oct 17. Psychiatry Clin Neurosci. 2023. PMID: 36148558 Free PMC article. Review.
Cited by
- Persistent isolated impairment of gas transfer following COVID-19 pneumonitis relates to perfusion defects on dual-energy computed tomography.
Price LC, Garfield B, Bloom C, Jeyin N, Nissan D, Hull JH, Patel B, Jenkins G, Padley S, Man W, Singh S, Ridge CA. Price LC, et al. ERJ Open Res. 2022 Nov 28;8(4):00224-2022. doi: 10.1183/23120541.00224-2022. eCollection 2022 Oct. ERJ Open Res. 2022. PMID: 36447736 Free PMC article. - Mechanisms of long COVID and the path toward therapeutics.
Peluso MJ, Deeks SG. Peluso MJ, et al. Cell. 2024 Oct 3;187(20):5500-5529. doi: 10.1016/j.cell.2024.07.054. Epub 2024 Sep 25. Cell. 2024. PMID: 39326415 Review. - Improvements during long-term fasting in patients with long COVID - a case series and literature review.
Grundler F, Mesnage R, Cerrada A, Wilhelmi de Toledo F. Grundler F, et al. Front Nutr. 2023 Nov 2;10:1195270. doi: 10.3389/fnut.2023.1195270. eCollection 2023. Front Nutr. 2023. PMID: 38024352 Free PMC article. - Long COVID: mechanisms, risk factors and recovery.
Astin R, Banerjee A, Baker MR, Dani M, Ford E, Hull JH, Lim PB, McNarry M, Morten K, O'Sullivan O, Pretorius E, Raman B, Soteropoulos DS, Taquet M, Hall CN. Astin R, et al. Exp Physiol. 2023 Jan;108(1):12-27. doi: 10.1113/EP090802. Epub 2022 Nov 22. Exp Physiol. 2023. PMID: 36412084 Free PMC article. Review. - Balancing the value and risk of exercise-based therapy post-COVID-19: a narrative review.
Singh SJ, Daynes E, McAuley HJC, Raman B, Greening NJ, Chalder T, Elneima O, Evans RA, Bolton CE. Singh SJ, et al. Eur Respir Rev. 2023 Dec 20;32(170):230110. doi: 10.1183/16000617.0110-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 38123233 Free PMC article. Review.
References
- Gerotziafas GT, Catalano M, Colgan MP, Pecsvarady Z, Wautrecht JC, Fazeli B, Olinic DM, Farkas K, Elalamy I, Falanga A, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European independent foundation in angiology/vascular medicine. Thromb Haemost. 2020;120(12):1597–1628. doi: 10.1055/s-0040-1715798. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous