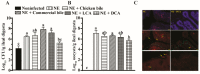Specific Secondary Bile Acids Control Chicken Necrotic Enteritis - PubMed (original) (raw)
Specific Secondary Bile Acids Control Chicken Necrotic Enteritis
Mohit Bansal et al. Pathogens. 2021.
Abstract
Necrotic enteritis (NE), mainly induced by the pathogens of Clostridium perfringens and coccidia, causes huge economic losses with limited intervention options in the poultry industry. This study investigated the role of specific bile acids on NE development. Day-old broiler chicks were assigned to six groups: noninfected, NE, and NE with four bile diets of 0.32% chicken bile, 0.15% commercial ox bile, 0.15% lithocholic acid (LCA), or 0.15% deoxycholic acid (DCA). The birds were infected with Eimeria maxima at day 18 and C. perfringens at day 23 and 24. The infected birds developed clinical NE signs. The NE birds suffered severe ileitis with villus blunting, crypt hyperplasia, epithelial line disintegration, and massive immune cell infiltration, while DCA and LCA prevented the ileitis histopathology. NE induced severe body weight gain (BWG) loss, while only DCA prevented NE-induced BWG loss. Notably, DCA reduced the NE-induced inflammatory response and the colonization and invasion of C. perfringens compared to NE birds. Consistently, NE reduced the total bile acids in the ileal digesta, while dietary DCA and commercial bile restored it. Together, this study showed that DCA and LCA reduced NE histopathology, suggesting that secondary bile acids, but not total bile acid levels, play an essential role in controlling the enteritis.
Keywords: Clostridium perfringens; Eimeria maxima; intestinal inflammation; microbiota; secondary bile acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Figure 1
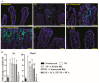
DCA Prevented Clinical NE-induced Ileal Inflammation. Cohorts of 7 to 14 broiler chickens were fed basal diets supplemented with different bile acids starting from day 14. Birds were challenged with E. maxima at day 18 and C. perfringens at day 23 and 24. The birds were sacrificed on day 25. (A) Representative images of H&E staining showing small intestinal histopathology. (B) Microscopic quantification of intestinal histopathological lesion score. Yellow arrows: immune cell infiltration; Green arrow: blunted villi; blue arrow: hyperplastic crypts. Different letters of a, b, and c mean p < 0.05. The scale bar is 200 μm. Results are representative of 3 independent experiments.
Figure 2
Cohorts of broiler chickens were fed different bile diets and infected as in Figure 1. (A) Representative images of the TUNEL assay showing cell death at the late stage of apoptosis (green dots). Yellow arrowhead: apoptotic immune cells in lamina propria; Green arrow: apoptotic epithelial cells. (B) Ileal Ifnγ and the Mmp9 mRNA qPCR fold-change relative to noninfected birds and normalized to Gapdh. Different letters of a and b mean p < 0.05. The scale bar is 40 μm. Results are representative of 3 independent experiments.
Figure 3
DCA reduced C. perfringens and E. maxima ileal colonization. Cohorts of broiler chickens were fed different bile diets and infected as in Figure 1. (A) Luminal C. perfringens colonization was quantified in the ileal digesta by measuring 16S rDNA using a qPCR. (B) Luminal E. maxima was quantified in the ileal digesta by measuring 18S rDNA using a qPCR. (C) C. perfringens mucosal invasion was visualized by FISH. Yellow arrows indicate the vegetative and spore cells (red) of C. perfringens. Yellow arrowheads indicate red blood cells (red). The blue color is the DAPI staining for the cell nucleus. The scale bar is 20 µm. All graphs show mean ± SEM. Different letters of a, b, and c mean p < 0.05. Results are representative of 3 independent experiments.
Figure 4
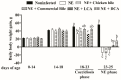
DCA reduced NE-induced productivity loss. Cohorts of broiler chickens were fed different bile supplemented diets from day 14 and infected as in Figure 1. Bird body weight gain (BWG) was measured at 0, 14, 18, and 25 days of age, and daily periodic BWG is shown. All graphs show mean ± SEM. Different letters of a, b, and c mean p < 0.05. Results are representative of 3 independent experiments.
Figure 5
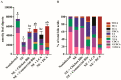
Dietary bile acids modulated the ileal bile acid composition in NE birds. Cohorts of broiler chickens fed with different bile acids and infected with NE as in Figure 1. (A) Total and individual bile acid quantification. (B) Relative composition of bile acids. All graphs show mean ± SEM. Different letters of a, b, and c mean p < 0.05. Results are representative of 3 independent experiments.
Similar articles
- Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis.
Kim DM, Liu J, Whitmore MA, Tobin I, Zhao Z, Zhang G. Kim DM, et al. J Anim Sci Biotechnol. 2024 Mar 2;15(1):29. doi: 10.1186/s40104-024-00995-9. J Anim Sci Biotechnol. 2024. PMID: 38429856 Free PMC article. - A secondary bile acid from microbiota metabolism attenuates ileitis and bile acid reduction in subclinical necrotic enteritis in chickens.
Bansal M, Fu Y, Alrubaye B, Abraha M, Almansour A, Gupta A, Liyanage R, Wang H, Hargis B, Sun X. Bansal M, et al. J Anim Sci Biotechnol. 2020 Mar 13;11:37. doi: 10.1186/s40104-020-00441-6. eCollection 2020. J Anim Sci Biotechnol. 2020. PMID: 32190299 Free PMC article. - Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling.
Wang H, Latorre JD, Bansal M, Abraha M, Al-Rubaye B, Tellez-Isaias G, Hargis B, Sun X. Wang H, et al. Sci Rep. 2019 Oct 10;9(1):14541. doi: 10.1038/s41598-019-51104-0. Sci Rep. 2019. PMID: 31601882 Free PMC article. - Subclinical Doses of Combined Fumonisins and Deoxynivalenol Predispose _Clostridium perfringens-_Inoculated Broilers to Necrotic Enteritis.
Shanmugasundaram R, Adams D, Ramirez S, Murugesan GR, Applegate TJ, Cunningham S, Pokoo-Aikins A, Glenn AE. Shanmugasundaram R, et al. Front Physiol. 2022 Jul 22;13:934660. doi: 10.3389/fphys.2022.934660. eCollection 2022. Front Physiol. 2022. PMID: 35936897 Free PMC article. - Comparison of the Pathogenicity of Five Clostridium perfringens Isolates Using an Eimeria maxima Coinfection Necrotic Enteritis Disease Model in Commercial Broiler Chickens.
Liu L, Yan X, Lillehoj H, Sun Z, Zhao H, Xianyu Z, Lee Y, Melville S, Gu C, Wang Y, Lu M, Li C. Liu L, et al. Avian Dis. 2020 Sep 1;64(3):386-392. doi: 10.1637/aviandiseases-D-19-00098. Avian Dis. 2020. PMID: 33205165
Cited by
- Effects of Dietary Limosilactobacillus fermentum and Lacticaseibacillus paracasei Supplementation on the Intestinal Stem Cell Proliferation, Immunity, and Ileal Microbiota of Broiler Chickens Challenged by Coccidia and Clostridium perfringens.
Guo S, Tong W, Qi Y, Jiang M, Li P, Zhang Z, Hu Q, Song Z, Ding B. Guo S, et al. Animals (Basel). 2023 Dec 15;13(24):3864. doi: 10.3390/ani13243864. Animals (Basel). 2023. PMID: 38136901 Free PMC article. - Vaccines Using Clostridium perfringens Sporulation Proteins Reduce Necrotic Enteritis in Chickens.
Fu Y, Bansal M, Alenezi T, Almansour A, Wang H, Sun X. Fu Y, et al. Microorganisms. 2022 May 27;10(6):1110. doi: 10.3390/microorganisms10061110. Microorganisms. 2022. PMID: 35744628 Free PMC article. - Potent Bile Acid Microbial Metabolites Modulate Clostridium perfringens Virulence.
Alenezi T, Fu Y, Alrubaye B, Alanazi T, Almansour A, Wang H, Sun X. Alenezi T, et al. Pathogens. 2023 Sep 28;12(10):1202. doi: 10.3390/pathogens12101202. Pathogens. 2023. PMID: 37887718 Free PMC article. - Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis.
Kim DM, Liu J, Whitmore MA, Tobin I, Zhao Z, Zhang G. Kim DM, et al. J Anim Sci Biotechnol. 2024 Mar 2;15(1):29. doi: 10.1186/s40104-024-00995-9. J Anim Sci Biotechnol. 2024. PMID: 38429856 Free PMC article. - Eimeria: Navigating complex intestinal ecosystems.
Weng S, Tian E, Gao M, Zhang S, Yang G, Zhou B. Weng S, et al. PLoS Pathog. 2024 Nov 22;20(11):e1012689. doi: 10.1371/journal.ppat.1012689. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39576763 Free PMC article. Review.
References
- Wang H., Latorre J.D., Bansal M., Abraha M., Al-Rubaye B., Tellez-Isaias G., Hargis B., Sun X. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci. Rep. 2019;9:14541. doi: 10.1038/s41598-019-51104-0. - DOI - PMC - PubMed
- Bansal M., Fu Y., Alrubaye B., Abraha M., Almansour A., Gupta A., Liyanage R., Wang H., Hargis B., Sun X. A secondary bile acid from microbiota metabolism attenuates ileitis and bile acid reduction in subclinical necrotic enteritis in chickens. J. Anim. Sci. Biotechnol. 2020;11:37. doi: 10.1186/s40104-020-00441-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials