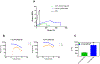Vascular Grafts with Tailored Stiffness and a Ligand Environment via Multiarmed Polymer Sheath for Expeditious Regeneration - PubMed (original) (raw)
Vascular Grafts with Tailored Stiffness and a Ligand Environment via Multiarmed Polymer Sheath for Expeditious Regeneration
Monica Iglesias-Echevarria et al. ACS Appl Bio Mater. 2021.
Abstract
The bypass graft is the mainstream of surgical intervention to treat vascular diseases. Ideal bypass materials, yet to be developed, require mechanical properties, availability, clinically feasible manufacturing logistics, and bioactivities with precise physicochemical cues defined to guide cell activities for arterial regeneration. Such needs instigated our fabrication of vascular grafts, which consist of coaxial, nanostructured fibers exhibiting a polycaprolactone (PCL) core and a photoclickable, 4-arm thiolated polyethylene glycol-norbornene (PEG-NB) sheath. The graft strength and bioactivity were modulated by the PCL concentration and the peptides (RGD, transforming growth factor _β_-1 or TGF-_β_1) conjugated to thiol-ene of PEG-NB, respectively. Structural, physical, and mechanical characterizations demonstrated that the fibrous grafts mimicked the key features of the native extracellular matrix, including a crosslinked fiber network for structural stability, viscoelasticity emulating arteries, hydration property, and high porosity for cell infiltration. Meanwhile, these grafts displayed strength and toughness exceeding or meeting surgical criteria. Furthermore, the grafts with higher PCL concentration (3 vs 1.8%) showed thicker fibers, lower porosity and pore size, and increased elastic and storage moduli. Graft bioactivity was determined by the mesenchymal stem cell (MSC) behaviors on the grafts and arterial regeneration in vivo using interposition grafting. Results showed that the cell adhesion and proliferation increased with the RGD density (25 vs 5 mM). After 1 week implantation, all peptide-functionalized PCL/PEG-NB grafts with or without MSC preseeding, as opposed to PCL grafts, showed expeditious endothelial lining, abundant vascular cell infiltration, and matrix production. Compared to RGD grafts, RGD/TGF-_β_1 grafts enhanced MSC differentiation into smooth muscle cells in vitro and developed thicker smooth muscle cell layers in vivo. Overall, the versatile porous vascular grafts offer superior properties and tunability for future translation.
Keywords: coaxial electrospun fiber; multi-armed polymer; polyethylene glycol-norbornene; regeneration; stiffness; vascular graft.
Figures
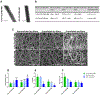
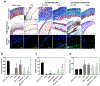
Figure 1.
Illustration of coaxial hybrid fibers imaged with electron microscopes and quantitatively evaluated based on the images. (A) TEM images of coaxially-electrospun fibers. Scale bar = 500 nm. (B) Quantitative analysis of the fiber micro/nanostructures. (C) SEM images of the uncrosslinked and crosslinked coaxial fiber scaffolds, which were measured in dry or wet states. Scale bar = 10 _μ_m. (D) Fiber diameter of the uncrosslinked and crosslinked coaxial fibers, before and after hydration. (E,F) Porosity (E) and pore size (F) of the uncrosslinked and crosslinked coaxial fibers, before and after hydration. “*”: p < 0.05 compared to dry uncrosslinked, “^”: p < 0.05 compared to dry crosslinked, and “#”: p < 0.05 compared to 1.8% PCL/PEG-NB. Additional significance levels were set at p < 0.01 (“**”, “^^” or “##”), p < 0.001 (“***”, “^^^” or “###”), and p < 0.0001 (“****”, “^^^^” or “####”).
Figure 2.
Tensile and viscoelastic characteristics of PCL/PEG-NB coaxially spun fibrous grafts. (A) Illustration of stress–strain curves for coaxial 1.8% and 3% PCL/PEG-NB fiber grafts, as well as PCL fiber graft. (B) Illustration of strain sweep results, showing G′ and G′′ obtained from the rheometer measurements of 1.8 and 3% PCL/PEG-NB grafts. (C) Storage modulus (G′) of grafts composed of 1.8% or 3% PCL/PEG-NB coaxial fibers. “**”: p < 0.01 compared to 1.8% PCL/PEG-NB.
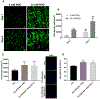
Figure 3.
Cell compatibility of the coaxial fiber grafts. (A) In vitro evaluation of hMSC attachment (day 1) and proliferation (day 4) on the coaxial fiber grafts, assessing different RGD concentrations. Fluorescent images display F-actin stain (in green) in hMSCs cultured for 24 and 96 h on the grafts consisting of PCL/PEG-NB. Results demonstrate preferential cell attachment and proliferation on the grafts treated with 25 mM RGD. Images were taken at 10×. Scale bar = 100 _μ_m. (B) Cell density on coaxial grafts treated with different RGD concentrations, measured at day 1 and day 4. “**”: p < 0.01 compared to 5 mM, “****”: p < 0.0001 compared to 5 mM, “##”: p < 0.01 compared to day 1. (C) Cell density on PCL/PEG-NB grafts functionalized with RGD or RGD + TGF-_β_1, as well as PCL fiber graft, measured at day 4. Fluorescent images display DAPI stain (in blue) in hMSCs cultured for 4 days on these grafts. (D) CCK assay of the cell cultures on the coaxial fiber grafts demonstrating higher viability of cells cultured on the coaxial fiber grafts compared to PCL graft. The absorbances were taken at 450 nm 96 h after cell seeding. “*” p < 0.05 and “**” p < 0.01, compared to PCL graft.
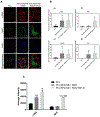
Figure 4.
Cell differentiation in the coaxial fiber vascular grafts. (A) Confocal microscopy images showing _α_-SMA and MHC stains in hMSCs cultured for 6 days on scaffolds made of PCL fibers, PCL/PEG-NB + RGD, and PCL/PEG-NB + RGD/TGF-_β_1. DAPI stain (blue) showing cell nuclei is shown as well. Images were taken at 10×. Scale bar = 50 _μ_m. (B) Gene expression on fiber scaffolds by qPCR showing _α_-SMA (a), SM-MHC (b), elastin (c), and collagen I (d). GAPDH was used as the reference house-keeping gene. (C) Average intensity calculated as the total mean grey value divided by the total number of cells from confocal images of the scaffolds. “*” p < 0.05, “**” p < 0.01, “***” p < 0.001, compared to PCL. “###” p < 0.001 compared to PCL/PEG-NB + RGD.
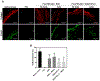
Figure 5.
Biological performances of vascular grafts. (A) Histological analyses, displaying cell infiltration and ECM production in the explanted grafts, as well as smooth muscle and endothelial layers. Masson’s trichrome stain displays collagen and mucus (blue), cell nuclei (black), and cell cytoplasm, keratin, muscle, and intercellular fiber (red). _α_-SMA/hematoxylin stain shows smooth muscle cells in brown, and cell nuclei in purple. The space between green dotted lines denotes _α_-SMA layer in implanted vascular grafts. For native artery, it denotes the smooth muscle layer or tunica media. vWF/DAPI stain shows cell nuclei in blue, and endothelial cell in green. “L” denotes the lumen. “D” denotes graft delamination. Scale bar = 100 _μ_m. (B) The cell penetration, (C) smooth muscle cell layer thickness, and (D) graft wall thickness measured on the explanted grafts. Significance comparisons include: “*” compared to PCL, “$” compared to RGD + rMSCs, “#” compared to RGD/TGF-_β_1, and “^” compared to RGD.
Figure 6.
ECM production measured in the anastomosis site of the explanted vascular grafts. (A) Multiphoton imaging of the grafts. Images display the produced collagen in red (from SHG imaging) and elastin in green (from TPE imaging). The area between white dotted lines shows the implanted vascular grafts. For native artery, it shows the area between tunica intima and adventitia. “L” represents the lumen. Scale bar = 100 _μ_m. (B) The ECM production measured on the explanted grafts. “*” Compared to PCL.
Similar articles
- Coaxial PCL/PEG-thiol-ene microfiber with tunable physico-chemical properties for regenerative scaffolds.
Iglesias-Echevarria M , Durante L , Johnson R , Rafuse M , Ding Y , Bonani W , Maniglio D , Tan W . Iglesias-Echevarria M , et al. Biomater Sci. 2019 Aug 20;7(9):3640-3651. doi: 10.1039/c9bm00388f. Biomater Sci. 2019. PMID: 31165794 Free PMC article. - Combinatorial screening of 3D biomaterial properties that promote myofibrogenesis for mesenchymal stromal cell-based heart valve tissue engineering.
Usprech J, Romero DA, Amon CH, Simmons CA. Usprech J, et al. Acta Biomater. 2017 Aug;58:34-43. doi: 10.1016/j.actbio.2017.05.044. Epub 2017 May 19. Acta Biomater. 2017. PMID: 28532900 - Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelial or smooth muscle cell markers.
Wingate K, Bonani W, Tan Y, Bryant SJ, Tan W. Wingate K, et al. Acta Biomater. 2012 Apr;8(4):1440-9. doi: 10.1016/j.actbio.2011.12.032. Epub 2012 Jan 8. Acta Biomater. 2012. PMID: 22266031 Free PMC article. - Coaxially-structured fibres with tailored material properties for vascular graft implant.
Johnson R, Ding Y, Nagiah N, Monnet E, Tan W. Johnson R, et al. Mater Sci Eng C Mater Biol Appl. 2019 Apr;97:1-11. doi: 10.1016/j.msec.2018.11.036. Epub 2018 Nov 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30678891 Free PMC article. - Polyglycerol sebacate-based elastomeric materials for arterial regeneration.
Wang Z, Zhang M, Liu L, Mithieux SM, Weiss AS. Wang Z, et al. J Biomed Mater Res A. 2024 Apr;112(4):574-585. doi: 10.1002/jbm.a.37583. Epub 2023 Jun 22. J Biomed Mater Res A. 2024. PMID: 37345954 Review.
Cited by
- Applying Principles of Regenerative Medicine to Vascular Stent Development.
Selvakumar PP, Rafuse MS, Johnson R, Tan W. Selvakumar PP, et al. Front Bioeng Biotechnol. 2022 Mar 7;10:826807. doi: 10.3389/fbioe.2022.826807. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35321023 Free PMC article. Review. - Matrix stiffness exacerbates the proinflammatory responses of vascular smooth muscle cell through the DDR1-DNMT1 mechanotransduction axis.
Wang J, Xie SA, Li N, Zhang T, Yao W, Zhao H, Pang W, Han L, Liu J, Zhou J. Wang J, et al. Bioact Mater. 2022 Jan 14;17:406-424. doi: 10.1016/j.bioactmat.2022.01.012. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386458 Free PMC article. - Strategies to counteract adverse remodeling of vascular graft: A 3D view of current graft innovations.
Tan W, Boodagh P, Selvakumar PP, Keyser S. Tan W, et al. Front Bioeng Biotechnol. 2023 Jan 10;10:1097334. doi: 10.3389/fbioe.2022.1097334. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36704297 Free PMC article. Review. - Redefining vascular repair: revealing cellular responses on PEUU-gelatin electrospun vascular grafts for endothelialization and immune responses on in vitro models.
Rodríguez-Soto MA, Riveros-Cortés A, Orjuela-Garzón IC, Fernández-Calderón IM, Rodríguez CF, Vargas NS, Ostos C, Camargo CM, Cruz JC, Kim S, D'Amore A, Wagner WR, Briceño JC. Rodríguez-Soto MA, et al. Front Bioeng Biotechnol. 2024 Jun 5;12:1410863. doi: 10.3389/fbioe.2024.1410863. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38903186 Free PMC article.
References
- Criqui MH; Aboyans V Epidemiology of Peripheral Artery Disease. Circ. Res 2015, 116, 1509–1526. - PubMed
- Cameron RB Bioengineered Vascular Grafts: So Close and yet so Far. J. Thorac. Cardiovasc. Surg 2018, 156, 1823–1824. - PubMed
- Dimitrievska S; Wang J; Lin T; Weyers A; Bai H; Qin L; Li G; Cai C; Kypson A; Kristofik N; Gard A; Sundaram S; Yamamoto K; Wu W; Zhao L; Kural MH; Yuan Y; Madri J; Kyriakides TR; Linhardt RJ; Niklason LE Glycocalyx-Like Hydrogel Coatings for Small Diameter Vascular Grafts. Adv. Funct. Mater 2020, 30, 1908963.
- Luo J; Qin L; Zhao L; Gui L; Ellis MW; Huang Y; Kural MH; Clark JA; Ono S; Wang J; Yuan Y; Zhang S-M; Cong X; Li G; Riaz M; Lopez C; Hotta A; Campbell S; Tellides G; Dardik A; Niklason LE; Qyang Y Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human IPSCs. Cell Stem Cell 2020, 26, 251–261. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources