Gut barrier and microbiota changes with glycine and branched-chain amino acid supplementation in chronic haemodialysis patients - PubMed (original) (raw)
Randomized Controlled Trial
. 2021 Dec;12(6):1527-1539.
doi: 10.1002/jcsm.12781. Epub 2021 Sep 18.
Menno Pruijm 2, Daniel Teta 3, Isabelle Bassi 3, Patrice D Cani 4, Nadia Gaïa 5, François R Herrmann 6, Nicola Marangon 7, Julie Mareschal 1, Giulio G Muccioli 8, Catherine Stoermann 9, Francesco Suriano 4, Arlene Wurzner-Ghajarzadeh 2, Vladimir Lazarevic 5, Jacques Schrenzel 5
Affiliations
- PMID: 34535959
- PMCID: PMC8718035
- DOI: 10.1002/jcsm.12781
Randomized Controlled Trial
Gut barrier and microbiota changes with glycine and branched-chain amino acid supplementation in chronic haemodialysis patients
Laurence Genton et al. J Cachexia Sarcopenia Muscle. 2021 Dec.
Abstract
Background: We have previously shown that glycine increases fat-free mass in chronic haemodialysis patients with features of malnutrition as compared with branched-chain amino acids (BCAAs). This multicentre randomized double-blind crossover study evaluates the impact of these amino acids on the gut barrier and microbiota.
Methods: Haemodialysis patients were included if they had plasma albumin <38 g/L or weight loss >5% of dry body weight, and daily dietary intakes <30 kcal/kg and <1 g protein/kg. They consumed glycine or BCAA (7 g twice daily) for 4 months and underwent a 1 month washout period, before crossover of supplementations. Faecal microbiota (16S rRNA gene sequencing) and immunoglobulin A (IgA), serum levels of cytokines, surrogate markers of intestinal permeability, appetite mediators, and endocannabinoids were obtained at the start and end of each supplementation. Supplementations were compared by multiple mixed linear regression models, adjusted for age, sex, month of supplementation (0 and 4 in each period), and period (Period 1: first 4 months; Period 2: last 4 months). Microbiota comparisons were performed using principal coordinate analysis and permutational multivariate analysis of variance, Shannon diversity index estimate and analysis of composition of microbiomes analysis, and Wilcoxon tests.
Results: We analysed 27 patients compliant to the supplementations. Multiple mixed linear regression models were significant only for interleukin-6 (P = 0.002), glucagon-like peptide 1 (P = 0.028), cholecystokinin (P = 0.021), and peptide YY (P = 0.002), but not for the other outcomes. The significant models did not show any impact of the type of supplementation (P < 0.05 in all models). Principal coordinate analysis and permutational multivariate analysis of variance (P = 0.0001) showed strong microbiota clustering by subject, but no effect of the amino acids. Bacterial alpha diversity and zero-radius operational taxonomic unit richness remained stable, whatever the supplementation. Lacticaseibacillus paracasei (0.030; Q1-Q3 0.008-0.078 vs. 0.004; Q1-Q3 0.001-0.070) and Bifidobacterium dentium (0.0247; Q1-Q3 0.002-0.191 vs. 0.003; Q1-Q3 0.001-0.086) significantly decreased with the BCAA supplementation.
Conclusions: The BCAA and glycine supplementations had no impact on the serum levels of cytokines, appetite mediators, intestinal permeability, endocannabinoids, or faecal IgA. Overall faecal microbiota composition and microbial diversity did not change with the glycine or BCAA supplementation but decreased the abundance of L. paracasei and B. dentium.
Trial registration: ClinicalTrials.gov NCT02962089.
Keywords: Appetite; Branched-chain amino acid malnutrition; Endocannabinoids; Glycine; Gut microbiota.
© 2021 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
L.G. has received grants from the Swiss National Foundation, Alfred and Alice Lachmann Foundation, and Fresenius Kabi; speaker honoraria from Fresenius Kabi; advisory honoraria from Baxter and Abbott; and travel grants from Nestlé Health Science and Abbott. P.D.C. is a co‐founder of A‐Mansia Biotech S.A. (Belgium) and owner of several patents concerning the use of specific bacteria or components on the treatment of obesity, diabetes, and cardiometabolic disorders. D.T. has received grants from Fresenius Medical Care, Amgen, and Baxter; speaker honoraria from Fresenius Medical Care, B Braun, Abbott International, Baxter, Genzyme, and Sanofi Aventis; travel grants for Fresenius Medical Care, Amgen, and Vifor; and a grant for teaching material from Abbott International. M.P., C.S., N.M., J.M., I.B., A.W.‐G., V.L., L.G., F.R.H., and J.S. have no conflicts of interest.
Figures
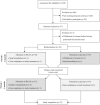
Figure 1
Consort flow diagram. BCAA, branched‐chain amino acid.
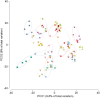
Figure 2
Principal coordinate analysis of Bray–Curtis similarity of bacterial communities showing that microbiota strongly cluster by subject. The analysis was based on the square‐root‐transformed relative abundance of zero‐radius operational taxonomic units. Four faecal samples were collected for each patient, at Months 0 and 4 of the glycine supplementation and Months 0 and 4 of the branched‐chain amino acid (BCAA) supplementation. As the overall microbiota similarity did not differ significantly between time points and supplementation, we omitted this legend on the figure for more clarity. The open symbols correspond to the samples of patients in the glycine–BCAA group and the filled symbols to the samples of patients in the BCAA–glycine group.
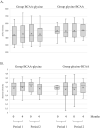
Figure 3
Alpha diversity of faecal bacterial communities. The dataset was rarefied to 41 500 reads prior to calculating (A) zero‐radius operational taxonomic unit (zOTU) richness and (B) Shannon diversity index; 0 and 4 indicate the sampling month. BCAA, branched‐chain amino acid.
Figure 4
Line plots showing the decrease of the relative abundance of Bifidobacterium dentium and Lacticaseibacillus paracasei, between Months 0 and 4, under branched‐chain amino acid (BCAA). Black lines represent the group treated first with BCAA in the first 4 months of the study and the grey lines the group treated with BCAA in the second 4 months of the study.
Similar articles
- Glycine increases fat-free mass in malnourished haemodialysis patients: a randomized double-blind crossover trial.
Genton L, Teta D, Pruijm M, Stoermann C, Marangon N, Mareschal J, Bassi I, Wurzner-Ghajarzadeh A, Lazarevic V, Cynober L, Cani PD, Herrmann FR, Schrenzel J. Genton L, et al. J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1540-1552. doi: 10.1002/jcsm.12780. Epub 2021 Sep 14. J Cachexia Sarcopenia Muscle. 2021. PMID: 34519439 Free PMC article. Clinical Trial. - Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis.
Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Hiroshige K, et al. Nephrol Dial Transplant. 2001 Sep;16(9):1856-62. doi: 10.1093/ndt/16.9.1856. Nephrol Dial Transplant. 2001. PMID: 11522870 Clinical Trial. - Diabetes and branched-chain amino acids: What is the link?
Bloomgarden Z. Bloomgarden Z. J Diabetes. 2018 May;10(5):350-352. doi: 10.1111/1753-0407.12645. Epub 2018 Feb 13. J Diabetes. 2018. PMID: 29369529 - Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review.
Ooi PH, Gilmour SM, Yap J, Mager DR. Ooi PH, et al. Clin Nutr ESPEN. 2018 Dec;28:41-51. doi: 10.1016/j.clnesp.2018.07.012. Epub 2018 Aug 14. Clin Nutr ESPEN. 2018. PMID: 30390892 - The effects of inulin on gut microbial composition: a systematic review of evidence from human studies.
Le Bastard Q, Chapelet G, Javaudin F, Lepelletier D, Batard E, Montassier E. Le Bastard Q, et al. Eur J Clin Microbiol Infect Dis. 2020 Mar;39(3):403-413. doi: 10.1007/s10096-019-03721-w. Epub 2019 Nov 9. Eur J Clin Microbiol Infect Dis. 2020. PMID: 31707507
Cited by
- Astragalus improves intestinal barrier function and immunity by acting on intestinal microbiota to treat T2DM: a research review.
Su M, Tang T, Tang W, Long Y, Wang L, Liu M. Su M, et al. Front Immunol. 2023 Aug 10;14:1243834. doi: 10.3389/fimmu.2023.1243834. eCollection 2023. Front Immunol. 2023. PMID: 37638043 Free PMC article. Review. - Glycine increases fat-free mass in malnourished haemodialysis patients: a randomized double-blind crossover trial.
Genton L, Teta D, Pruijm M, Stoermann C, Marangon N, Mareschal J, Bassi I, Wurzner-Ghajarzadeh A, Lazarevic V, Cynober L, Cani PD, Herrmann FR, Schrenzel J. Genton L, et al. J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1540-1552. doi: 10.1002/jcsm.12780. Epub 2021 Sep 14. J Cachexia Sarcopenia Muscle. 2021. PMID: 34519439 Free PMC article. Clinical Trial. - The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders.
Du C, Liu WJ, Yang J, Zhao SS, Liu HX. Du C, et al. Front Nutr. 2022 Jul 18;9:932670. doi: 10.3389/fnut.2022.932670. eCollection 2022. Front Nutr. 2022. PMID: 35923208 Free PMC article. Review. - Gut Microbiota-Targeted Interventions in the Management of Chronic Kidney Disease.
Sumida K, Pierre JF, Yuzefpolskaya M, Colombo PC, Demmer RT, Kovesdy CP. Sumida K, et al. Semin Nephrol. 2023 Mar;43(2):151408. doi: 10.1016/j.semnephrol.2023.151408. Epub 2023 Aug 22. Semin Nephrol. 2023. PMID: 37619529 Free PMC article. Review. - Gut microbiota associations with chronic kidney disease: insights into nutritional and inflammatory parameters.
Lazarevic V, Teta D, Pruijm M, Stoermann C, Marangon N, Mareschal J, Solano R, Wurzner-Ghajarzadeh A, Gaïa N, Cani PD, Dizdar OS, Herrmann FR, Schrenzel J, Genton L. Lazarevic V, et al. Front Microbiol. 2024 May 21;15:1298432. doi: 10.3389/fmicb.2024.1298432. eCollection 2024. Front Microbiol. 2024. PMID: 38835485 Free PMC article.
References
- Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, et al. Global Prevalence of Protein‐Energy Wasting in Kidney Disease: A Meta‐analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:380–392. - PubMed
- Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched‐chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant 2001;16:1856–1862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous