Inhibitory activity of FDA-approved drugs cetilistat, abiraterone, diiodohydroxyquinoline, bexarotene, remdesivir, and hydroxychloroquine on COVID-19 main protease and human ACE2 receptor: A comparative in silico approach - PubMed (original) (raw)
Inhibitory activity of FDA-approved drugs cetilistat, abiraterone, diiodohydroxyquinoline, bexarotene, remdesivir, and hydroxychloroquine on COVID-19 main protease and human ACE2 receptor: A comparative in silico approach
Nahid Shahabadi et al. Inform Med Unlocked. 2021.
Abstract
By September 1, 2021, SARS-CoV-2, a respiratory virus that prompted Coronavirus Disease in 2019, had infected approximately 218,567,442 patients and claimed 4,534,151 lives. There are currently no specific treatments available for this lethal virus, although several drugs, including remdesivir and hydroxychloroquine, have been tested. The purpose of this study is to assess the activity of FDA-approved drugs cetilistat, abiraterone, diiodohydroxyquinoline, bexarotene, remdesivir, and hydroxychloroquine as potential SARS-CoV-2 main protease inhibitors. Additionally, this study aims to provide insight into the development of potential inhibitors that may inhibit ACE2, thereby preventing SARS-CoV-2 entry into the host cell and infection. To this end, remdesivir and hydroxychloroquine were used as comparator drugs. The calculations revealed that cetilistat, abiraterone, diiodohydroxyquinoline, and bexarotene inhibit main protease and ACE2 receptors more effectively than the well-known drug hydroxychloroquine when used against COVID-19. Meanwhile, bexarotene and cetilistat bind more tightly to the SARS-CoV-2 main protease and the ACE2 receptor, respectively, than remdesivir, a potential treatment for COVID-19 that is the first FDA-approved drug against this virus. As a result, the molecular dynamic simulations of these two drugs in the presence of proteins were investigated. The MD simulation results demonstrated that these drugs interact to stabilize the systems, allowing them to be used as effective inhibitors of these proteins. Meanwhile, bexarotene, abiraterone, cetilistat, and diiodohydroxyquinoline's systemic effects should be further investigated in suitable ex vivo human organ culture or organoids, animal models, or clinical trials.
Keywords: ACE2 receptor; COVID-19; Dynamic simulation; Main protease; Molecular docking.
© 2021 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Graphical abstract
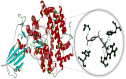
Fig. 1
Molecular docking perspective of abiraterone – (ACE2-RBD).
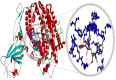
Fig. 2
Molecular docking perspective of bexarotene – (ACE2-RBD).
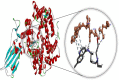
Fig. 3
Molecular docking perspective of cetilistat – (ACE2-RBD).
Fig. 4
Molecular docking perspective of diiodohydroxyquinoline – (ACE2-RBD).
Fig. 5
Molecular docking perspective of remdesivir – (ACE2-RBD).
Fig. 6
Molecular docking perspective of hydroxychloroquine – (ACE2-RBD).
Fig. 7
Radius of gyration (Rg) for (A) bexarotene – CoV-2 main protease and (B) cetilistat – (ACE2-RBD) during 30 ns MD simulation.
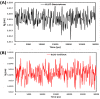
Fig. 8
RMSD plots for (A) bexarotene – CoV-2 main protease and (B) cetilistat – (ACE2-RBD) during 30 ns MD simulation.
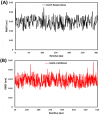
Fig. 9
RMSF plots for (A) bexarotene – CoV-2 main protease and (B) cetilistat – (ACE2-RBD) during 30 ns MD simulation.
Similar articles
- Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system.
Yuan S, Chan JFW, Chik KKH, Chan CCY, Tsang JOL, Liang R, Cao J, Tang K, Chen LL, Wen K, Cai JP, Ye ZW, Lu G, Chu H, Jin DY, Yuen KY. Yuan S, et al. Pharmacol Res. 2020 Sep;159:104960. doi: 10.1016/j.phrs.2020.104960. Epub 2020 May 28. Pharmacol Res. 2020. PMID: 32473310 Free PMC article. - Different compounds against Angiotensin-Converting Enzyme 2 (ACE2) receptor potentially containing the infectivity of SARS-CoV-2: an in silico study.
Shahbazi B, Mafakher L, Teimoori-Toolabi L. Shahbazi B, et al. J Mol Model. 2022 Mar 5;28(4):82. doi: 10.1007/s00894-022-05059-1. J Mol Model. 2022. PMID: 35249180 Free PMC article. - Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.
Uzunova K, Filipova E, Pavlova V, Vekov T. Uzunova K, et al. Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review. - Candidate drugs against SARS-CoV-2 and COVID-19.
McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. McKee DL, et al. Pharmacol Res. 2020 Jul;157:104859. doi: 10.1016/j.phrs.2020.104859. Epub 2020 Apr 29. Pharmacol Res. 2020. PMID: 32360480 Free PMC article. Review.
Cited by
- Coordination chemistry suggests that independently observed benefits of metformin and Zn2+ against COVID-19 are not independent.
Lockwood TD. Lockwood TD. Biometals. 2024 Aug;37(4):983-1022. doi: 10.1007/s10534-024-00590-5. Epub 2024 Apr 5. Biometals. 2024. PMID: 38578560 Free PMC article. - In Silico Models for Anti-COVID-19 Drug Discovery: A Systematic Review.
Onyango OH. Onyango OH. Adv Pharmacol Pharm Sci. 2023 Jun 15;2023:4562974. doi: 10.1155/2023/4562974. eCollection 2023. Adv Pharmacol Pharm Sci. 2023. PMID: 37362912 Free PMC article. Review. - Determination of Alteration in Micromeritic Properties of a Solid Dispersion: Brunauer-Emmett-Teller Based Adsorption and Other Structured Approaches.
Singh L, Kaur L, Singh G, Dhawan RK, Kaur M, Kaur N, Singh P. Singh L, et al. AAPS PharmSciTech. 2022 Jul 28;23(6):209. doi: 10.1208/s12249-022-02367-w. AAPS PharmSciTech. 2022. PMID: 35902454 Free PMC article. - Enzymes in the time of COVID-19: An overview about the effects in the human body, enzyme market, and perspectives for new drugs.
Fé LXSGM, Cipolatti EP, Pinto MCC, Branco S, Nogueira FCS, Ortiz GMD, Pinheiro AS, Manoel EA. Fé LXSGM, et al. Med Res Rev. 2022 Nov;42(6):2126-2167. doi: 10.1002/med.21919. Epub 2022 Jun 28. Med Res Rev. 2022. PMID: 35762498 Free PMC article. Review.
References
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. In press. - DOI
- Muralidharan N., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J Biomol Struct Dyn. 2020;39:2673–2678. - PubMed
- Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J Biomol Struct Dyn. 2020;39:2607–2616. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous