A Human Pleiotropic Multiorgan Condition Caused by Deficient Wnt Secretion - PubMed (original) (raw)
. 2021 Sep 30;385(14):1292-1301.
doi: 10.1056/NEJMoa2033911.
Emmanuelle Szenker-Ravi 1, Changuk Chung 1, Zhen Li 1, Lu Wang 1, Muznah Khatoo 1, Trevor Marshall 1, Nan Jiang 1, Xiaoxu Yang 1, Jennifer McEvoy-Venneri 1, Valentina Stanley 1, Paula Anzenberg 1, Nhi Lang 1, Vanessa Wazny 1, Jia Yu 1, David M Virshup 1, Rie Nygaard 1, Filippo Mancia 1, Rijad Merdzanic 1, Maria B P Toralles 1, Paula M L Pitanga 1, Ratna D Puri 1, Rebecca Hernan 1, Wendy K Chung 1, Aida M Bertoli-Avella 1, Nouriya Al-Sannaa 1, Maha S Zaki 1, Karl Willert 1, Bruno Reversade 1, Joseph G Gleeson 1
Affiliations
- PMID: 34587386
- PMCID: PMC9017221
- DOI: 10.1056/NEJMoa2033911
A Human Pleiotropic Multiorgan Condition Caused by Deficient Wnt Secretion
Guoliang Chai et al. N Engl J Med. 2021.
Abstract
Background: Structural birth defects occur in approximately 3% of live births; most such defects lack defined genetic or environmental causes. Despite advances in surgical approaches, pharmacologic prevention remains largely out of reach.
Methods: We queried worldwide databases of 20,248 families that included children with neurodevelopmental disorders and that were enriched for parental consanguinity. Approximately one third of affected children in these families presented with structural birth defects or microcephaly. We performed exome or genome sequencing of samples obtained from the children, their parents, or both to identify genes with biallelic pathogenic or likely pathogenic mutations present in more than one family. After identifying disease-causing variants, we generated two mouse models, each with a pathogenic variant "knocked in," to study mechanisms and test candidate treatments. We administered a small-molecule Wnt agonist to pregnant animals and assessed their offspring.
Results: We identified homozygous mutations in WLS, which encodes the Wnt ligand secretion mediator (also known as Wntless or WLS) in 10 affected persons from 5 unrelated families. (The Wnt ligand secretion mediator is essential for the secretion of all Wnt proteins.) Patients had multiorgan defects, including microcephaly and facial dysmorphism as well as foot syndactyly, renal agenesis, alopecia, iris coloboma, and heart defects. The mutations affected WLS protein stability and Wnt signaling. Knock-in mice showed tissue and cell vulnerability consistent with Wnt-signaling intensity and individual and collective functions of Wnts in embryogenesis. Administration of a pharmacologic Wnt agonist partially restored embryonic development.
Conclusions: Genetic variations affecting a central Wnt regulator caused syndromic structural birth defects. Results from mouse models suggest that what we have named Zaki syndrome is a potentially preventable disorder. (Funded by the National Institutes of Health and others.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Figure 1.
Homozygous Missense Mutations in WLS Leading to a Pleiotropic Recognizable Syndrome. Panel A shows pedigrees of five families with affected persons showing recessive inheritance. Homozygous WLS mutations and genotypes of available family members are indicated. Squares indicate male family members, circles female family members, double bars consanguinity, open symbols unaffected, black symbols affected, gray symbol likely affected, and diagonal slash deceased. The term wt denotes wild type, and mut mutant. Panel B shows images of affected persons. At left, sagittal T1-weighted magnetic resonance imaging of the brain in Patient F1-IV:4 shows reduced brain volume and an enlarged fourth ventricle (white arrowhead). In the three middle images, dysmorphic features include abnormal outer ears (black arrowhead), wide mouth, iris coloboma (Patient F2-IV:4, black arrow), and sparse scalp hair and eyebrows. At right, a “claw” malformation of the feet (double arrowheads) was observed in Patient F2-IV:4. We propose that this distinctive clinical presentation represents Zaki syndrome. Panel C shows the topologic features of WLS within the endoplasmic reticulum (ER) membrane. Patient mutations are represented with stars. Y392 and Y478 locate at the sixth and eighth transmembrane domain, respectively. I531 and R536 locate in the C-terminal tail, just before or within the ER-targeting signal, respectively. Panel D shows immunoblots of endogenous WLS protein in control and patient (F3-II:1) primary dermal fibroblasts. GAPDH denotes glyceraldehyde-3-phosphate dehydrogenase. Panel E shows immunoblots of overexpressed, untagged WLS in WLS knockout (KO) HEK293T cells. The p.Y392C and p.Y478C mutations lead to lower WLS protein levels than wild-type WLS.
Figure 2.
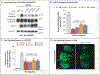
Effect of WLS Mutations on Wnt Secretion and Signaling. Panel A shows Western blot analysis of WNT3A in culture medium and lysates of WLS knockout (WLS_KO) HEK293T cells transfected with WNT3A and pcDNA3 or WLS. Panel B shows the ratio of immunoblots of secreted WNT3A to intracellular WNT3A, normalized to wild-type (WT) WLS. Panel C shows a Super TOPFlash assay from WLS_KO HEK293T cells transfected with Wnt3a and WT or mutant WLS plasmids cocultured with control HEK293T cells transfected with Super TOPFlash and Renilla reporter plasmids. RLU denotes relative light unit. The values in Panels B and C are means calculated by one-way analysis of variance; T bars indicate standard deviations. Panel D shows whole TCF/Lef:H2B-GFP transgenic mouse embryos at embryonic day 12.5 imaged with a light-sheet microscope, revealing globally lower Wnt signaling in WlsY478C/Y478C embryos than in controls. Arrowheads indicate the spinal cord. The term e denotes ear, fb forebrain, h heart, k kidney, m mouth, mb midbrain, nd nephric duct, sc spinal cord, and t tail. The scale bar indicates 1 mm.
Figure 3.
A Spectrum of Developmental Defects in Wls Knock-in Mouse Embryos. Panel A shows mouse skeletons at embryonic day 18.5 stained with Alcian blue (cartilage) and Alizarin red (bone). Wls knock-in embryos had defective caudal vertebrae (arrowhead). Panel B shows whole kidneys (top) and sections stained with hematoxylin and eosin (bottom) from WT, WlsY392C/Y392C, and WlsY478C/Y478C embryos with cysts (asterisk) at embryonic day 18.5. Panel C shows representative images of forelimbs and hindlimbs with reduced digit number in WlsY392C/Y392C embryos at embryonic day 14.5. Panel D shows images of whole brains and sections of such brains at embryonic day 18.5. The left column shows whole brains. In the middle column, Nissl-stained sections of brains show the regions of the hippocampus. Mutants showed defective dentate gyrus (DG) (black arrowhead). CA1 denotes cornu ammonis subfield 1, CA3 cornu ammonis subfield 3, and FF fimbria—fornix. In the right column, magnification of mouse cortex stained for CUX1 and CTIP2 shows defective cortical lamination and ectopias (arrowhead) in Wls mutants. DAPI denotes 4’,6-diamidino-2-phenylindole. Panel E shows quantification of cortical CUX1+ and CTIP2+ neurons in WT and Wls mutant embryos at embryonic day 18.5. Data are for 4 mice per genotype. Values are means calculated with a two-tailed, unpaired t-test, and T bars indicate standard deviations. Scale bars indicate 50 μm in the middle and right columns in Panel D and 1 mm elsewhere.
Figure 4.
Partial Rescue of Defects in Wls Knock-in Embryos by Wnt Agonist Treatment. Panel A shows the induced embryoid bodies from human induced pluripotent stem cells (iPSCs) at day 19. Panel B shows the quantification of the diameters of embryoid bodies derived from control and patient iPSCs, treated with dimethylsulfoxide (DMSO) or 2.5 μM of CHIR99021. Data are for 23 embryoid bodies from control and patient iPSCs treated with DMSO and 16 embryoid bodies from patient iPSCs treated with CHIR99021. Panel C shows the skeletons of embryos at embryonic day 18.5 stained with Alcian blue (cartilage) and Alizarin red (bone). Panel D shows the quantification of vertebrae, indicating rescued sacral and caudal vertebrae in Wls mutants by CHIR99021. Mice generally have 7 cervical, 13 thoracic, 6 lumbar, 4 sacral, and 28 caudal vertebrae. All embryos had a normal number of cervical and thoracic vertebrae, and the sums of lumbar, sacral, and caudal vertebrae were calculated. Data are for 5 WT mice treated with DMSO, 8 Wls mutants treated with DMSO, 6 WT mice treated with CHIR99021, and 6 Wls mutants treated with CHIR99021. Panel E shows images of whole kidneys (top) and brains (bottom) in embryos at embryonic day 18.5 from dams treated with DMSO or CHIR99021. Arrows indicate small kidneys, and arrowheads cystic kidneys with hydronephrosis. Each yellow line indicates a caliper for dorsoventral axis thickness. Panel F shows the percent of mutants with kidney phenotypes. Data are for 10 WT mice treated with DMSO, 16 mutants treated with DMSO, and 22 Wls mutants treated with CHIR99021. Panel G shows quantification of CUX1+ and CTIP2+ cortical neurons. A total of 4 WT mice and 5 Wls mutants were included for each group, with the left and right sides of the brain measured separately. In Panels B, D, and G, values are means calculated by one-way analysis of variance, and T bars or I bars indicate standard deviations. Scale bars indicate 500 μm in Panel A and 1 mm in Panels C and E.
Similar articles
- Expanding the phenotypic spectrum and clinical severity associated with WLS gene.
Abdel-Salam GMH, Afifi HH, Abdel-Hamid MS, Ahmed NEB, Taher MB, El-Kamah G, Thiele H, Nürnberg PN, Bolz HJ. Abdel-Salam GMH, et al. J Hum Genet. 2023 Sep;68(9):607-613. doi: 10.1038/s10038-023-01152-2. Epub 2023 Apr 28. J Hum Genet. 2023. PMID: 37106064 - Mesenchymal Wnt signaling promotes formation of sternum and thoracic body wall.
Snowball J, Ambalavanan M, Cornett B, Lang R, Whitsett J, Sinner D. Snowball J, et al. Dev Biol. 2015 May 15;401(2):264-75. doi: 10.1016/j.ydbio.2015.02.014. Epub 2015 Feb 26. Dev Biol. 2015. PMID: 25727890 Free PMC article. - Identification of an endocytosis motif in an intracellular loop of Wntless protein, essential for its recycling and the control of Wnt protein signaling.
Gasnereau I, Herr P, Chia PZ, Basler K, Gleeson PA. Gasnereau I, et al. J Biol Chem. 2011 Dec 16;286(50):43324-33. doi: 10.1074/jbc.M111.307231. Epub 2011 Oct 25. J Biol Chem. 2011. PMID: 22027831 Free PMC article. - Compound heterozygous variants in WLS gene causes Zaki syndrome.
Yu C, Wang C, Zhou W, Zhang A, Jia Z, Zheng B, Ding G. Yu C, et al. Clin Genet. 2023 Aug;104(2):226-229. doi: 10.1111/cge.14334. Epub 2023 Apr 2. Clin Genet. 2023. PMID: 37005218 - Wnt trafficking: new insights into Wnt maturation, secretion and spreading.
Port F, Basler K. Port F, et al. Traffic. 2010 Oct;11(10):1265-71. doi: 10.1111/j.1600-0854.2010.01076.x. Traffic. 2010. PMID: 20477987 Review.
Cited by
- Update on Biology and Genomics of Adrenocortical Carcinomas: Rationale for Emerging Therapies.
Lerario AM, Mohan DR, Hammer GD. Lerario AM, et al. Endocr Rev. 2022 Nov 25;43(6):1051-1073. doi: 10.1210/endrev/bnac012. Endocr Rev. 2022. PMID: 35551369 Free PMC article. - Molecular Mechanisms of circRNA-miRNA-mRNA Interactions in the Regulation of Goose Liver Development.
Liu S, Li C, Hu X, Mao H, Liu S, Chen B. Liu S, et al. Animals (Basel). 2024 Mar 8;14(6):839. doi: 10.3390/ani14060839. Animals (Basel). 2024. PMID: 38539937 Free PMC article. - Protein interaction networks characterizing the A549 cells Klotho transfected are associated with activated pro-apoptotic Bim and suppressed Wnt/β-catenin signaling pathway.
Matsumoto M, Ogawa N, Fukuda T, Bando Y, Nishimura T, Usuda J. Matsumoto M, et al. Sci Rep. 2024 Jan 25;14(1):2130. doi: 10.1038/s41598-024-52616-0. Sci Rep. 2024. PMID: 38267588 Free PMC article. - Spatial gene expression profile of Wnt-signaling components in the murine enteric nervous system.
Scharr M, Hirt B, Neckel PH. Scharr M, et al. Front Immunol. 2024 Jan 18;15:1302488. doi: 10.3389/fimmu.2024.1302488. eCollection 2024. Front Immunol. 2024. PMID: 38322254 Free PMC article. - Molecular basis of Wnt biogenesis, secretion, and Wnt7-specific signaling.
Qi X, Hu Q, Elghobashi-Meinhardt N, Long T, Chen H, Li X. Qi X, et al. Cell. 2023 Nov 9;186(23):5028-5040.e14. doi: 10.1016/j.cell.2023.09.021. Epub 2023 Oct 17. Cell. 2023. PMID: 37852257 Free PMC article.
References
- Agopian AJ, Evans JA, Lupo PJ. Analytic methods for evaluating patterns of multiple congenital anomalies in birth defect registries. Birth Defects Res 2018; 110:5–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 HG008900/HG/NHGRI NIH HHS/United States
- F32 NS009800/NS/NINDS NIH HHS/United States
- R01 NS048453/NS/NINDS NIH HHS/United States
- U54 HG006504/HG/NHGRI NIH HHS/United States
- R01 NS098004/NS/NINDS NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- P01 HD104436/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials