Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism - PubMed (original) (raw)
Randomized Controlled Trial
. 2022 Feb 10;43(6):518-533.
doi: 10.1093/eurheartj/ehab644.
Arash Haghikia 1 2 3, Paul Schumann 1 2, Andrzej Jasina 1, Johann Roessler 1 2, David Schmidt 1, Philipp Heinze 1, Johannes Kaisler 4, Vanasa Nageswaran 1, Annette Aigner 3 5, Uta Ceglarek 6 7, Roodline Cineus 8 9, Ahmed N Hegazy 3 8 9, Emiel P C van der Vorst 10 11 12 13, Yvonne Döring 10 11 14, Christopher M Strauch 15, Ina Nemet 15, Valentina Tremaroli 16, Chinmay Dwibedi 16 17, Nicolle Kränkel 1 2, David M Leistner 1 2 3, Markus M Heimesaat 18, Stefan Bereswill 18, Geraldine Rauch 3 5, Ute Seeland 2 19, Oliver Soehnlein 10 11 20, Dominik N Müller 2 3 21 22, Ralf Gold 4, Fredrik Bäckhed 16 23 24, Stanley L Hazen 15 25, Aiden Haghikia 26, Ulf Landmesser 1 2 3
Affiliations
- PMID: 34597388
- PMCID: PMC9097250
- DOI: 10.1093/eurheartj/ehab644
Randomized Controlled Trial
Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism
Arash Haghikia et al. Eur Heart J. 2022.
Abstract
Aims: Atherosclerotic cardiovascular disease (ACVD) is a major cause of mortality and morbidity worldwide, and increased low-density lipoproteins (LDLs) play a critical role in development and progression of atherosclerosis. Here, we examined for the first time gut immunomodulatory effects of the microbiota-derived metabolite propionic acid (PA) on intestinal cholesterol metabolism.
Methods and results: Using both human and animal model studies, we demonstrate that treatment with PA reduces blood total and LDL cholesterol levels. In apolipoprotein E-/- (Apoe-/-) mice fed a high-fat diet (HFD), PA reduced intestinal cholesterol absorption and aortic atherosclerotic lesion area. Further, PA increased regulatory T-cell numbers and interleukin (IL)-10 levels in the intestinal microenvironment, which in turn suppressed the expression of Niemann-Pick C1-like 1 (Npc1l1), a major intestinal cholesterol transporter. Blockade of IL-10 receptor signalling attenuated the PA-related reduction in total and LDL cholesterol and augmented atherosclerotic lesion severity in the HFD-fed Apoe-/- mice. To translate these preclinical findings to humans, we conducted a randomized, double-blinded, placebo-controlled human study (clinical trial no. NCT03590496). Oral supplementation with 500 mg of PA twice daily over the course of 8 weeks significantly reduced LDL [-15.9 mg/dL (-8.1%) vs. -1.6 mg/dL (-0.5%), P = 0.016], total [-19.6 mg/dL (-7.3%) vs. -5.3 mg/dL (-1.7%), P = 0.014] and non-high-density lipoprotein cholesterol levels [PA vs. placebo: -18.9 mg/dL (-9.1%) vs. -0.6 mg/dL (-0.5%), P = 0.002] in subjects with elevated baseline LDL cholesterol levels.
Conclusion: Our findings reveal a novel immune-mediated pathway linking the gut microbiota-derived metabolite PA with intestinal Npc1l1 expression and cholesterol homeostasis. The results highlight the gut immune system as a potential therapeutic target to control dyslipidaemia that may introduce a new avenue for prevention of ACVDs.
Keywords: Atherosclerosis; Gut microbiome; Propionic acid.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures
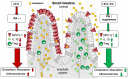
Graphical Abstract
The figure illustrates the proposed model of the cholesterol-lowering and atheroprotective properties of propionate. A high-fat high-cholesterol diet causes a disbalance of immune cells in the small intestinal microenvironment, with reduced regulatory T cell frequencies and interleukin-10 concentrations. Altered regulatory T cell levels are rescued upon exogenous propionate supplementation, with increased local levels. This in turn modulates NPC1L1 expression and membrane density, with a subsequent reduction in cholesterol absorption, ultimately leading to reduced atherogenesis. The illustration was adopted from Servier Medical Art (
), licensed under a Creative Common Attribution 3.0 Generic License. HFD, high-fat diet; IL-10, interleukin-10; NPC1L1, Niemann-Pick C1-like 1; PA, propionic acid; Treg, regulatory T cell.
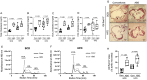
Figure 1
The gut microbiota regulates cholesterol metabolism and impacts atherogenesis. (A_–_D) HPLC analysis of blood levels of total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in conventionally raised (Conv, black circles) or antibiotic treated (ABS, white circles) _Apoe_−/− mice fed either a standard chow or a high-fat diet (Conv: standard chow n = 12, high-fat diet n = 10; ABS: standard chow n = 4, high-fat diet n = 7). (E and F) Representative HPLC-assisted fractionation of plasma lipids under a standard chow or a high-fat diet. (G and H) Representative images of Oil Red O-stained aortic root sections (scale bars represent 100 µm) with quantification of lipid deposition (Conv, black circles: standard chow n = 8, high-fat diet n = 8; ABS, white circles: standard chow n = 5, high-fat diet n = 6). Data were analysed by a two-tailed unpaired _t_-test between two groups. HDL, high-density lipoprotein; HFD, high-fat diet; HPLC, High-performance liquid chromatography; LDL, low-density lipoprotein; SFD, standard chow; VLDL, very low-density lipoprotein.
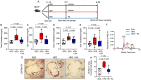
Figure 2
Propionate prevents high-fat diet-induced hypercholesterolaemia and atherosclerosis in _Apoe_−/− mice. (A) _Apoe_−/− mice were fed either a standard chow diet (standard chow, n = 12) or a high-fat diet (high-fat diet, n = 24) for 6 weeks. After 2 weeks, the standard chow-fed mice received sodium chloride (vehicle), and the high-fat diet-fed mice were treated with either propionate (propionic acid, n = 13) or sodium chloride (vehicle, n = 11) via oral gavage until the end of the experiment. (B_–_E) HPLC analysis of blood levels of total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in _Apoe_−/− mice at the end of the experiments (standard chow n = 12, high-fat diet n = 10, high-fat diet + propionic acid n = 13). (F) Representative HPLC-assisted fractionation of plasma lipids. (G and H) Representative images of Oil Red O-stained aortic root sections (scale bars represent 100 µm) with quantification of lipid deposition (standard chow n = 8, high-fat diet n = 8, high-fat diet + propionic acid n = 11). For the analysis in (B_–_H), the results of the Conv standard chow and Conv high-fat diet groups were the same results used in Figure 1. Data were analysed by one-way ANOVA followed by post hoc Tukey’s test. HDL, high-density lipoprotein; HFD, high-fat diet; LDL, low-density lipoprotein; PA, propionic acid; SFD, standard chow; VLDL, very low-density lipoprotein.
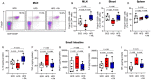
Figure 3
Effect of propionate on expression of genes involved in hepatic and intestinal cholesterol metabolism. (A_–_E) Expression of hepatic Srebp2, Cyp7a1, Ldlr, and small intestinal Npc1l1 and Asbt, as assessed by quantitative polymerase chain reaction at the end of the treatment (standard chow n = 11–12, high-fat diet n = 10–11 and high-fat diet + propionic acid n = 12–13). (F) Representative immunostaining (scale bars represent 100 µm) of the small intestine of mice using an NPC1L1 antibody (upper row, immunohistochemistry; lower row, immunofluorescence) with (G) quantification of the mean fluorescence intensity (standard chow n = 9, high-fat diet n = 7 and high-fat diet + propionic acid n = 9). (H and I) Analysis of blood levels of the phytosterols stigmasterol and sitosterol at the end of the experiments (standard chow n = 11, high-fat diet n = 10–11, high-fat diet + propionic acid n = 13). Data were analysed by one-way ANOVA followed by post hoc Tukey’s test. HFD, high-fat diet; PA, propionic acid; SFD, standard chow.
Figure 4
Treatment with propionate increases regulatory T cells and interleukin-10 in the small intestine. (A) Representative FACS plots of regulatory T cells in the mesenteric lymph nodes from mice in the standard chow, high-fat diet and high-fat diet + propionic acid groups. (B and C) Compared to the standard chow (n = 7), the high-fat diet (n = 7) reduced regulatory T cells in the mesenteric lymph nodes and peripheral blood, and this change was reversed by propionic acid treatment (n = 7–8). (D) Regulatory T cells in the spleen were not altered by high-fat diet or propionic acid. (E_–_I) Cytokine analysis of the small intestine revealed an increase in interleukin-10 levels after treatment with propionic acid, whereas the levels of tumour necrosis factor-alpha, monocyte chemoattractant protein-1, interleukin-6 and interferon gamma were not altered by propionic acid. Data were analysed by one-way ANOVA followed by post hoc Tukey’s test. HFD, high-fat diet; IFN-γ, interferon gamma; IL-10, interleukin-10; MCP-1, monocyte chemoattractant protein-1; MLN, mesenteric lymph node; PA, propionic acid; SFD, standard chow; TNF-α, tumour necrosis factor-alpha.
Figure 5
Regulation of Npc1l1 gene expression by interleukin-10 mediates propionate-dependent control of intestinal cholesterol absorption and atheroprotection. (A) Representative photomicrograph of a mouse small intestinal epithelial organoid. (B) The organoids express both the interleukin-10R1 and interleukin-10R2 receptors, as demonstrated by reverse transcription-polymerase chain reaction. (C) Treatment of the organoids with murine recombinant interleukin-10 down-regulated the expression of the Npc1l1 gene in a dose-dependent manner (n = 4 independent biological replicates). (D) Interleukin-10 signalling was inhibited by weekly i.p. injection of a monoclonal blocking antibody against the interleukin-10 receptor in high-fat diet-fed _Apoe_−/− mice during the propionic acid treatment period (high-fat diet + propionic acid n = 13, high-fat diet + propionic acid + interleukin-10R antibody n = 8). (E_–_H) HPLC analysis of blood levels of total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in high-fat diet + propionic acid mice in the presence (grey) or absence (blue) of interleukin-10R antibody. (I) Representative HPLC-assisted fractionation of plasma lipids. (J and K) Representative images of Oil Red O-stained aortic root sections (scale bars represent 100 µm) with quantification of lipid deposition (high-fat diet + propionic acid n = 11, high-fat diet + propionic acid + interleukin-10R antibody n = 7). (L and M) Analysis of blood levels of the phytosterols stigmasterol and sitosterol at the end of the experiments (high-fat diet + propionic acid n = 13, high-fat diet + propionic acid + interleukin-10R antibody n = 8). For the analysis in E_–_M, the results for the high-fat diet + propionic acid group were the same results used in Figure 2. Data were analysed by a two-tailed unpaired _t_-test. HDL, high-density lipoprotein; HFD, high-fat diet; LDL, low-density lipoprotein; IL-10, interleukin-10; PA, propionic acid; VLDL, very low-density lipoprotein.
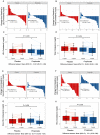
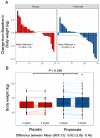
Figure 6
Oral propionate supplementation lowers blood low-density lipoprotein and total cholesterol levels in hypercholesterolaemic humans. (A and B) Waterfall plots depict the change in low-density lipoprotein and total cholesterol levels a from baseline to Week 8 for each study participant randomly assigned to the placebo group and the propionic acid group. (C and D) Box plots illustrate the distribution of low-density lipoprotein and total cholesterol levels within the placebo (red) and propionate (blue) groups for each timepoint (baseline and after 8 weeks). (E and F) Waterfall plots showing the change in non-high-density lipoprotein and high-density lipoprotein cholesterol levels. (G and H) Box plots demonstrating the distribution of non-high-density lipoprotein and high-density lipoprotein cholesterol levels for each timepoint. The lines depict the raw values at baseline and after 8 weeks. In the box plots, the line in the middle of the box indicates the median, and the lower and upper limits of the box correspond to the 25th and 75th percentiles, respectively. Analysis of covariance was performed to test the delta change between the two groups (placebo n = 29, propionate n = 29). CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Figure 7
Effect of oral propionate supplementation on body weight. (A) Waterfall plots depict the change in body weight from baseline to Week 8 for each study participant randomly assigned to the placebo group and the propionic acid group. (B) Box plots illustrate the distribution of body weight within the placebo (red) and propionate (blue) groups for each timepoint (baseline and after 8 weeks). The lines depict the raw values at baseline and after 8 weeks. In the box plots, the line in the middle of the box indicates the median, and the lower and upper limits of the box correspond to the 25th and 75th percentiles, respectively. Analysis of covariance was performed to test the delta change between the two groups (placebo n = 29, propionate n = 29). CI, confidence interval; SD, standard deviation;
Comment in
- The promise of the gut metabolite propionate for a novel and personalized lipid-lowering treatment.
Osto E. Osto E. Eur Heart J. 2022 Feb 10;43(6):534-537. doi: 10.1093/eurheartj/ehab723. Eur Heart J. 2022. PMID: 34686871 Free PMC article. No abstract available.
Similar articles
- Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice.
Zou J, Zhang S, Li P, Zheng X, Feng D. Zou J, et al. Nutr Res. 2018 Aug;56:32-40. doi: 10.1016/j.nutres.2018.04.017. Epub 2018 May 3. Nutr Res. 2018. PMID: 30055772 - Lycopene Reduces Cholesterol Absorption and Prevents Atherosclerosis in ApoE-/- Mice by Downregulating HNF-1α and NPC1L1 Expression.
Liu H, Liu J, Liu Z, Wang Q, Liu J, Feng D, Zou J. Liu H, et al. J Agric Food Chem. 2021 Sep 8;69(35):10114-10120. doi: 10.1021/acs.jafc.1c03160. Epub 2021 Aug 25. J Agric Food Chem. 2021. PMID: 34428895 - Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE-/- mice.
Davis HR Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Davis HR Jr, et al. Arterioscler Thromb Vasc Biol. 2007 Apr;27(4):841-9. doi: 10.1161/01.ATV.0000257627.40486.46. Epub 2007 Jan 11. Arterioscler Thromb Vasc Biol. 2007. PMID: 17218600 - Cholesterol homeostasis by the intestine: lessons from Niemann-Pick C1 Like 1 [NPC1L1).
Davis HR Jr, Basso F, Hoos LM, Tetzloff G, Lally SM, Altmann SW. Davis HR Jr, et al. Atheroscler Suppl. 2008 Sep;9(2):77-81. doi: 10.1016/j.atherosclerosissup.2008.05.008. Epub 2008 Jun 27. Atheroscler Suppl. 2008. PMID: 18585981 Review. - Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter.
Davis HR Jr, Altmann SW. Davis HR Jr, et al. Biochim Biophys Acta. 2009 Jul;1791(7):679-83. doi: 10.1016/j.bbalip.2009.01.002. Epub 2009 Jan 19. Biochim Biophys Acta. 2009. PMID: 19272334 Review.
Cited by
- Chinese Herbal Medicines for Coronary Heart Disease: Clinical Evidence, Pharmacological Mechanisms, and the Interaction with Gut Microbiota.
Cao L, Ni H, Gong X, Zang Z, Chang H. Cao L, et al. Drugs. 2024 Feb;84(2):179-202. doi: 10.1007/s40265-024-01994-w. Epub 2024 Jan 24. Drugs. 2024. PMID: 38265546 Review. - Probiotics Bring New Hope for Atherosclerosis Prevention and Treatment.
Zhai T, Wang P, Hu X, Zheng L. Zhai T, et al. Oxid Med Cell Longev. 2022 Sep 24;2022:3900835. doi: 10.1155/2022/3900835. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193065 Free PMC article. Review. - The Role of the Gut Microbiome and Trimethylamine Oxide in Atherosclerosis and Age-Related Disease.
El Hage R, Al-Arawe N, Hinterseher I. El Hage R, et al. Int J Mol Sci. 2023 Jan 25;24(3):2399. doi: 10.3390/ijms24032399. Int J Mol Sci. 2023. PMID: 36768722 Free PMC article. Review. - Short-chain fatty acids in diseases.
Zhang D, Jian YP, Zhang YN, Li Y, Gu LT, Sun HH, Liu MD, Zhou HL, Wang YS, Xu ZX. Zhang D, et al. Cell Commun Signal. 2023 Aug 18;21(1):212. doi: 10.1186/s12964-023-01219-9. Cell Commun Signal. 2023. PMID: 37596634 Free PMC article. Review. - High Throughput Fabrication of Flexible Top-Driven Sensing Probe.
Li F, Liu X, Wang W, Xu H, Song W, Sun Z. Li F, et al. Polymers (Basel). 2022 Nov 24;14(23):5124. doi: 10.3390/polym14235124. Polymers (Basel). 2022. PMID: 36501518 Free PMC article.
References
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M.. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. - PubMed
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. - PubMed
- Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall-Pedoe H, Moitry M, Iacoviello L, Veronesi G, Grassi G, Mathiesen EB, Söderberg S, Linneberg A, Brenner H, Amouyel P, Ferrières J, Tamosiunas A, Nikitin YP, Drygas W, Melander O, Jöckel KH, Leistner DM, Shaw JE, Panagiotakos DB, Simons LA, Kavousi M, Vasan RS, Dullaart RPF, Wannamethee SG, Risérus U, Shea S, de Lemos JA, Omland T, Kuulasmaa K, Landmesser U, Blankenberg S, Zeller T, Kontto J, Männistö S, Metspalu A, Lackner K, Wild P, Peters A, Meisinger C, Donfrancesco C, Signorini SG, Alver M, Woodward M, Gianfagna F, Costanzo S, Wilsgaard T, Eliasson M, Jørgensen T, Völzke H, Dörr M, Nauck M, Schöttker B, Lorenz T, Makarova N, Twerenbold R, Dallongeville J, Dobson A, Malyutina S, Pajak A, Engström G, Bobak M, Schmidt B, Jääskeläinen T, Niiranen T, Jousilahti P, Giles G, Hodge A, Klotsche J, Magliano DJ, Lyngbakken MN, Hveem K, Pitsavos C, Benjamin EJ, Bakker SJL, Whincup P, Ikram MK, Ingelsson M, Koenig W.. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet 2019;394:P2173–P2183. - PMC - PubMed
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL.. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous