The neurogliovascular unit in hepatic encephalopathy - PubMed (original) (raw)
Review
The neurogliovascular unit in hepatic encephalopathy
Wouter Claeys et al. JHEP Rep. 2021.
Abstract
Hepatic encephalopathy (HE) is a neurological complication of hepatic dysfunction and portosystemic shunting. It is highly prevalent in patients with cirrhosis and is associated with poor outcomes. New insights into the role of peripheral origins in HE have led to the development of innovative treatment strategies like faecal microbiota transplantation. However, this broadening of view has not been applied fully to perturbations in the central nervous system. The old paradigm that HE is the clinical manifestation of ammonia-induced astrocyte dysfunction and its secondary neuronal consequences requires updating. In this review, we will use the holistic concept of the neurogliovascular unit to describe central nervous system disturbances in HE, an approach that has proven instrumental in other neurological disorders. We will describe HE as a global dysfunction of the neurogliovascular unit, where blood flow and nutrient supply to the brain, as well as the function of the blood-brain barrier, are impaired. This leads to an accumulation of neurotoxic substances, chief among them ammonia and inflammatory mediators, causing dysfunction of astrocytes and microglia. Finally, glymphatic dysfunction impairs the clearance of these neurotoxins, further aggravating their effect on the brain. Taking a broader view of central nervous system alterations in liver disease could serve as the basis for further research into the specific brain pathophysiology of HE, as well as the development of therapeutic strategies specifically aimed at counteracting the often irreversible central nervous system damage seen in these patients.
Keywords: ABC, ATP-binding cassette; ACLF, acute-on-chronic liver failure; AD, acute decompensation; ALF, acute liver failure; AOM, azoxymethane; AQP4, aquaporin 4; Acute Liver Failure; Ammonia; BBB, blood-brain barrier; BCRP, breast cancer resistance protein; BDL, bile duct ligation; Blood-brain barrier; Brain edema; CCL, chemokine ligand; CCR, C-C chemokine receptor; CE, cerebral oedema; CLD, chronic liver disease; CLDN, claudin; CNS, central nervous system; CSF, cerebrospinal fluid; Cirrhosis; Energy metabolism; GS, glutamine synthetase; Glymphatic system; HE, hepatic encephalopathy; HO-1, heme oxygenase 1; IL-, interleukin; MMP-9, matrix metalloproteinase 9; MRP, multidrug resistance associated protein; NGVU; NGVU, neurogliovascular unit; NKCC1, Na-K-2Cl cotransporter 1; Neuroinflammation; OCLN, occludin; ONS, oxidative and nitrosative stress; Oxidative stress; P-gp, P-glycoprotein; PCA, portacaval anastomosis; PSS, portosystemic shunt; S1PR2, sphingosine-1-phosphate receptor 2; SUR1, sulfonylurea receptor 1; Systemic inflammation; TAA, thioacetamide; TGFβ, transforming growth factor beta; TJ, tight junction; TNF, tumour necrosis factor; TNFR1, tumour necrosis factor receptor 1; ZO, zonula occludens; mPT, mitochondrial pore transition.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
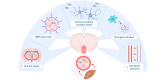
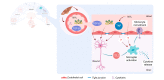
Fig. 1
The neurogliovascular unit in hepatic encephalopathy. In patients with decompensated cirrhosis, circulating levels of cytokines, ammonia and bile acids are elevated and reach the brain. In patients with hepatic encephalopathy, cerebral blood flow and nutrient supply are impaired. The BBB becomes leaky, allowing toxins like ammonia to enter the brain. Ammonia induces astrocyte swelling and oxidative stress, impairing normal functioning and eventually resulting in brain oedema. Microglia are activated and produce inflammatory mediators, resulting in a neuroinflammatory environment, further aggravating neurogliovascular dysfunction. Finally, impaired glymphatic clearance results in the accumulation of neurotoxic compounds. BBB, blood-brain barrier; Gln, glutamine; NH3, ammonia; ROS, reactive oxygen species.
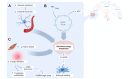
Fig. 2
Energy metabolism is impaired. (A) In hepatic encephalopathy, vascular resistance is increased in the cerebral circulation, leading to decreased blood flow and thus supply of oxygen and nutrients to the brain. (B) Additionally, ammonia inhibits key Krebs cycle enzymes like alpha-ketoglutarate dehydrogenase, leading to ATP depletion. (C) Inflammation leads to preferential energy and nutrient allocation towards the innate immune system. Ketone body production by the cirrhotic liver as an alternative energy source for the brain is insufficient. Additionally, toxic compounds like kynurenines and GABA precursors accumulate secondary to increased amino acid metabolism. Lactate, the end product of glycolysis, accumulates in the brain and might act as an osmolyte, leading to astrocyte swelling and brain oedema. GABA, gamma amino butyric acid; NH3, ammonia.
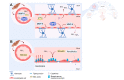
Fig. 3
Blood-brain barrier structure and function is altered in hepatic encephalopathy. (A) In (chronic) liver disease, levels of circulating ammonia, bile acids, cytokines, MMP-9 and TGFβ1 are increased. MMP-9 degrades TJ proteins like occludin and claudin-5. TGFβ1 leads to additional MMP-9 production by cerebral endothelial cells. Bile acids lead to deficient formation of TJs. Finally, cytokines like TNF decrease expression of TJ proteins like occludin. TJ breakdown leads to indiscriminate influx of toxins like ammonia. (B) Efflux transporters like BCRP protect the brain from drugs and other xenobiotics. Ammonia and bilirubin, which accumulate in conditions of HE, reduce expression and functionality of BCRP. This increases brain concentrations of xenobiotics and other ligands of BCRP and makes patients more susceptible to these compounds. BBB, blood-brain barrier; BCRP, breast cancer resistance protein; HE, hepatic encephalopathy; MMP-9, matrix metalloproteinase 9; NH3, ammonia; TGFβ, transforming growth factor beta; TJ, tight junction; TNF, tumour necrosis factor.
Fig. 4
Astrocytes swell and are subject to oxidative stress in hepatic encephalopathy. (A) Ammonia is metabolised into glutamine by glutamine synthetase. Glutamine acts as an osmolyte. Alternatively, glutamine is shuttled into the mitochondria, leading to mitochondrial pore transition and oxidative stress. Both mechanisms contribute to astrocyte swelling and cerebral oedema. (B) Astrocyte exposure to excess ammonia leads to overactivation of NKCC1 and the SUR1-regulated NCCa-ATP channel, resulting in influx of ions into the cell. Water follows the gradient created through increased AQP4 water channels, finally resulting in astrocyte swelling and cerebral oedema. (C) Ammonia leads to Ca2+ influx, activation of NADPH oxidase and mitochondrial pore transition. Tryptophan metabolites might also induce free radical production. These mechanisms increase levels of ROS, which enters a positive feedback loop with astrocyte swelling. Additionally, it leads to astrocyte senescence, possibly explaining the irreversible symptoms of HE. AQP4, aquaporin 4; Gln, glutamine; HE, hepatic encephalopathy; NADPH, nicotinamide adenine dinucleotide phosphate; NH3, ammonia; NKCC1, Na-K-2Cl cotransporter 1; ROS, reactive oxygen species; SUR1, sulfonylurea receptor 1.
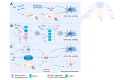
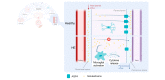
Fig. 5
Mechanisms and consequences of microglial activation in hepatic encephalopathy. In (chronic) liver disease, circulating levels of ammonia, TCA, TGFβ1 and cytokines like TNF are increased. TGFβ1, TNF and TCA bind to their respective neuronal receptors, which respond with CCL2 production. CCL2 binds to CCR2/4 on microglia, resulting in an activated phenotype. Additionally, hyperammonaemia can induce peripheral TNF increases, which induces microglial activation through the TNFR1, although whether this effect is direct or indirect is not known. Whether compounds like bile acids and cytokines have a direct influence on microglia in HE is unknown. The direct effect of hyperammonaemia on microglia is still an open question. Microglial activation results in cytokine production and recruitment of peripheral immune cells, in particular monocytes. This constitutes a neuroinflammatory environment, which is detrimental to brain functioning. CCL, chemokine ligand; CCR, C-C chemokine receptor; NH3, ammonia; TCA, taurocholic acid; TGFβ, transforming growth factor beta; TNF, tumour necrosis factor; TNFR1, TNF receptor 1.
Fig. 6
Glymphatic dysfunction leads to accumulation of toxins and neuroinflammation. In chronic liver disease, AQP4 vessel coverage is decreased. This impairs the flow of solutes and toxins from the periarterial to the perivenous space and further on to meningeal lymphatic vessels. Subsequently, these compounds accumulate in the brain parenchyma, activating microglia and promoting a neuroinflammatory microenvironment, which disturbs normal brain functioning. AQP4, aquaporin 4; HE, hepatic encephalopathy.
Similar articles
- Pathogenesis of Hepatic Encephalopathy in Chronic Liver Disease.
Ochoa-Sanchez R, Rose CF. Ochoa-Sanchez R, et al. J Clin Exp Hepatol. 2018 Sep;8(3):262-271. doi: 10.1016/j.jceh.2018.08.001. Epub 2018 Aug 18. J Clin Exp Hepatol. 2018. PMID: 30302043 Free PMC article. Review. - Hepatic Encephalopathy: Current Thoughts on Pathophysiology and Management.
Sen BK, Pan K, Chakravarty A. Sen BK, et al. Curr Neurol Neurosci Rep. 2025 Mar 28;25(1):28. doi: 10.1007/s11910-025-01415-9. Curr Neurol Neurosci Rep. 2025. PMID: 40153081 Review. - Neuroinflammation in hepatic encephalopathy: mechanistic aspects.
Jayakumar AR, Rama Rao KV, Norenberg MD. Jayakumar AR, et al. J Clin Exp Hepatol. 2015 Mar;5(Suppl 1):S21-8. doi: 10.1016/j.jceh.2014.07.006. Epub 2014 Aug 5. J Clin Exp Hepatol. 2015. PMID: 26041953 Free PMC article. Review. - Blood-brain barrier dysfunction in hepatic encephalopathy: pathophysiology, diagnostic assessment and therapeutic perspectives.
Le Guennec L, Mouri S, Thabut D, Weiss N. Le Guennec L, et al. Metab Brain Dis. 2025 Jun 13;40(5):223. doi: 10.1007/s11011-025-01645-3. Metab Brain Dis. 2025. PMID: 40512384 Review. - Diacerein ameliorates thioacetamide-induced hepatic encephalopathy in rats via modulation of TLR4/AQP4/MMP-9 axis.
Abd Elrazik NA, Abd El Salam ASG. Abd Elrazik NA, et al. Metab Brain Dis. 2024 Nov 18;40(1):10. doi: 10.1007/s11011-024-01457-x. Metab Brain Dis. 2024. PMID: 39556255 Free PMC article.
Cited by
- Experimental hepatic encephalopathy causes early but sustained glial transcriptional changes.
Claeys W, Van Hoecke L, Lernout H, De Nolf C, Van Imschoot G, Van Wonterghem E, Verhaege D, Castelein J, Geerts A, Van Steenkiste C, Vandenbroucke RE. Claeys W, et al. J Neuroinflammation. 2023 May 29;20(1):130. doi: 10.1186/s12974-023-02814-w. J Neuroinflammation. 2023. PMID: 37248507 Free PMC article. - Regulation of the blood-brain barrier function by peripheral cues in health and disease.
Devraj K, Kulkarni O, Liebner S. Devraj K, et al. Metab Brain Dis. 2024 Dec 13;40(1):61. doi: 10.1007/s11011-024-01468-8. Metab Brain Dis. 2024. PMID: 39671124 Free PMC article. Review. - A mouse model of hepatic encephalopathy: bile duct ligation induces brain ammonia overload, glial cell activation and neuroinflammation.
Claeys W, Van Hoecke L, Geerts A, Van Vlierberghe H, Lefere S, Van Imschoot G, Van Wonterghem E, Ghesquière B, Vandenbroucke RE, Van Steenkiste C. Claeys W, et al. Sci Rep. 2022 Oct 20;12(1):17558. doi: 10.1038/s41598-022-22423-6. Sci Rep. 2022. PMID: 36266427 Free PMC article. - Navigating the Labyrinth: Intensive Care Challenges for Patients with Acute-on-Chronic Liver Failure.
Saner FH, Raptis DA, Alghamdi SA, Malagó MM, Broering DC, Bezinover D. Saner FH, et al. J Clin Med. 2024 Jan 16;13(2):506. doi: 10.3390/jcm13020506. J Clin Med. 2024. PMID: 38256640 Free PMC article. Review. - Design of a Highly Active Peptide Inhibitor of Farnesyltransferase and Its Protective Effect Against Acute Liver Failure.
Huang CL, Qu HS, Li AL, Ying CQ, Shao H, Tang YZ, Chen HZ, Tung TH, Zhu JS. Huang CL, et al. Drug Des Devel Ther. 2025 Mar 13;19:1909-1926. doi: 10.2147/DDDT.S505541. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40098903 Free PMC article.
References
- Bustamante J., Rimola A., Ventura P.-J., Navasa M., Cirera I., Reggiardo V. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999 May;30(5):890–895. - PubMed
- Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014 Sep;61(3):642–659. - PubMed
- Tapper E.B., Jiang Z.G., Patwardhan V.R. Refining the ammonia hypothesis. Mayo Clin Proc. 2015 May;90(5):646–658. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous